Currently the treatment of choice for critical liver failure is liver transplantation. Liver failure is treated conservatively until a matching liver donor becomes available. The therapeutic plasma exchange (TPE) plays an important role as a bridge to transplantation by removing accumulated toxins from patient plasma, as well as restoring the coagulation profile.
MethodThis was a retrospective study on critically ill liver disease patients who underwent TPE from January 2012 to September 2015. The data were collected for the analyses of coagulation parameters, liver function tests, renal function tests, model for end-stage liver disease (MELD) scores, mortality, and hospital stay.
ResultsIn the study duration, a total of 45 patients with critical liver disease underwent therapeutic plasma exchange. The TPE resulted in a statistically significant reduction in the bilirubin level, aspartate aminotransferase (AST), alanine aminotransferase (ALT), prothrombin time (PT), international normalized ratio (INR), serum ferritin level and MELD scores. Higher MELD scores in both pre- and post-TPE were associated with higher mortality during the hospital stay.
ConclusionThe TPE is safe and well-tolerated, and it improves coagulation profile and liver function tests in critically ill liver disease patients, but the overall survival remains low.
The liver is involved in critical bodily functions including synthesis of proteins, coagulation factors, and clearance of biliary excretions and toxic metabolites. Liver failure puts patients at risk of coagulopathy and spontaneous hemorrhages, usually in the brain tissues. Accumulation of toxic substances such as bilirubin, aromatic amino acids, benzodiazepines, and ammonia further make patients more vulnerable to develop cerebral edema.1 Liver failure is caused by severe and widespread hepatocyte dysfunction and is associated with a high mortality rate of up to 60–80% with conservative treatment. Toxin accumulation in the plasma leads to hepatic encephalopathy which adds to the poor prognosis in these patients.2 The patients are initially managed conservatively by giving optimal nutrition, blood component therapy for coagulopathy and anemia, antibiotics to prevent infections and other symptomatic supportive therapy. Conservative treatment of liver failure is less effective than liver transplantation, but due to a shortage of liver donors, second-line therapies become the practical treatment of choice. Currently, purification of the blood using artificial liver support systems is becoming popular and has shown some clinical efficacy in liver disease patients. Among the liver support systems, the most widely used therapies are the therapeutic plasma exchange (TPE), and the molecular adsorbent recirculating system (MARS) treatments.3 The TPE supports the failing liver by removing the accumulated toxins in plasma, improving the coagulation profile by replacing coagulation factors and maintaining the fluid balance.1,4 As per the guidelines established by the American Society for Apheresis (ASFA) in 2016: “TPE in acute liver failure is a category III recommendation, while in fulminant hepatic failure with hemolysis in Wilson's disease it is a category I recommendation”.4 The TPE provides supportive care until spontaneous recovery occurs or a liver allograft becomes available for liver transplantation. The effectiveness of the TPE in this scenario, as the treatment of choice for the maintenance of patients awaiting liver transplantation, is yet to be ascertained. We aimed to analyze the outcomes of the TPE procedures performed at our center in critically ill liver disease patients by determining the improvement in clinical and laboratory parameters.
MethodologyThis retrospective observational study was performed at a tertiary care center for liver diseases in North India from January 2012 to September 2015. Ethical approval for the study was obtained from the Institute's ethics committee. All the patients awaiting liver transplantation who underwent therapeutic plasma exchange during the study period were included. Patients who developed sepsis or multiorgan dysfunction prior to the TPE and patients with the category IV indication, as per the 2016 ASFA guidelines, were excluded from the study. All TPE procedures were performed by a transfusion medicine specialist, along with the critical care team, using the Spectra Optia Apheresis System (Terumo BCT Inc., Lakewood, CO, USA), at the liver intensive care unit. In each procedure, approximately one plasma volume was exchanged, as per the clinical indication and the requisition from the treating clinician. The TPE procedures were performed every day, using fresh frozen plasma (FFP) as the main replacement fluid. The acid-citrate-dextrose (ACD) anticoagulant was used in a ratio of 1:14 with whole blood throughout the procedure. A 12 French femoral catheter was used for venous access. Supplementary calcium was given in the form of calcium gluconate, 10ml in 10% w/v, with normal saline, using an infusion pump over the period of 20–30min for every 800–1000ml of replacement fluid to prevent citrate toxicity. Venous blood gas (VBG) was used to monitor calcium levels and additional calcium gluconate was provided, as needed. Pre-procedural and 12h post-procedural laboratory parameters, such as platelet count, coagulation parameters including prothrombin time (PT), international normalized ratio (INR), liver function tests (total, direct and indirect bilirubin level), aspartate transaminase (AST), and alanine transaminase (ALT), serum ferritin and serum creatinine, were recorded. The requirements of vasopressors and dose adjustment of the same during the TPE were also recorded. The MELD (model for end-stage liver disease) score was calculated for the assessment of outcomes, with its clinical correlation. The laboratory parameters and MELD scores were expressed as mean±SD in patients. The mortality analysis was performed using the chi-square test, and pre- and post-TPE treatment values were assessed using the Student t-test. A p-value of less than 0.05 was considered statistically significant, wherever applicable.
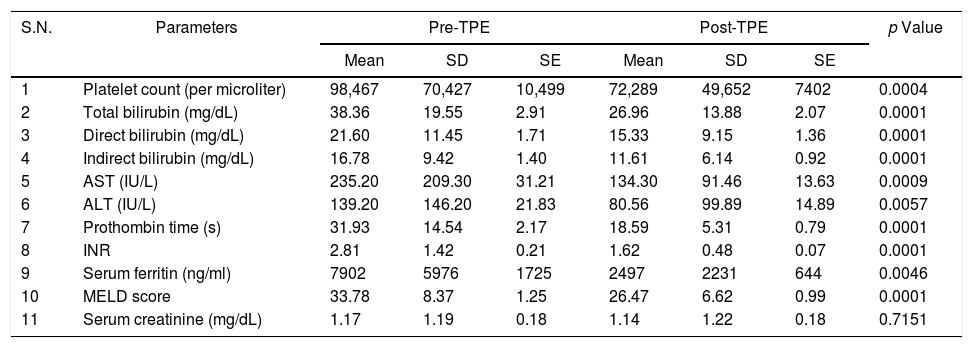
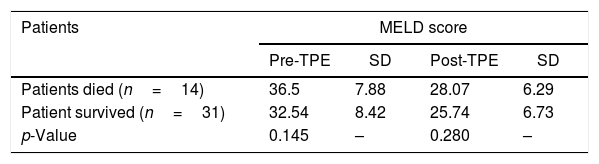
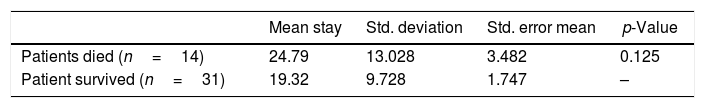
ResultsDuring the study period, a total of 45 patients with critical liver disease underwent therapeutic plasma exchange. The mean age of the patients was 37.75 years. In the study, 69% (31) of the patients were males, and 31% (14), females. All the procedures were performed daily, with a range of 2–6 procedures (mean of 2.8 procedures) per patient. A statistically significant reduction was observed in all the post-procedural values, such as total, direct and indirect bilirubin, AST, ALT, PT, INR, serum ferritin level and MELD score (p<0.05), with the exception of serum creatinine levels (p>0.05), as shown in Table 1. The platelet count fell during the procedure. The mean arterial pressure fluctuated during the procedure in most of the patients and required dose adjustment of noradrenaline and dopamine. The patients who had higher MELD scores, both pre- and post-TPE, had a higher mortality during their hospital stay, but this was not statistically significant (p>0.05), as shown in Table 2. A total of 14 patients died during their hospital stay and the major cause of death was septic shock, associated with multi-organ failure. The duration of the hospital stay was longer for patients who succumbed, as compared to that of those who survived, but this was not significant (p=0.125), as shown in Table 3. No procedure-related complications were observed during the TPE, except for hypocalcemia.
Pre- and post-TPE laboratory parameters.
| S.N. | Parameters | Pre-TPE | Post-TPE | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Mean | SD | SE | |||
| 1 | Platelet count (per microliter) | 98,467 | 70,427 | 10,499 | 72,289 | 49,652 | 7402 | 0.0004 |
| 2 | Total bilirubin (mg/dL) | 38.36 | 19.55 | 2.91 | 26.96 | 13.88 | 2.07 | 0.0001 |
| 3 | Direct bilirubin (mg/dL) | 21.60 | 11.45 | 1.71 | 15.33 | 9.15 | 1.36 | 0.0001 |
| 4 | Indirect bilirubin (mg/dL) | 16.78 | 9.42 | 1.40 | 11.61 | 6.14 | 0.92 | 0.0001 |
| 5 | AST (IU/L) | 235.20 | 209.30 | 31.21 | 134.30 | 91.46 | 13.63 | 0.0009 |
| 6 | ALT (IU/L) | 139.20 | 146.20 | 21.83 | 80.56 | 99.89 | 14.89 | 0.0057 |
| 7 | Prothombin time (s) | 31.93 | 14.54 | 2.17 | 18.59 | 5.31 | 0.79 | 0.0001 |
| 8 | INR | 2.81 | 1.42 | 0.21 | 1.62 | 0.48 | 0.07 | 0.0001 |
| 9 | Serum ferritin (ng/ml) | 7902 | 5976 | 1725 | 2497 | 2231 | 644 | 0.0046 |
| 10 | MELD score | 33.78 | 8.37 | 1.25 | 26.47 | 6.62 | 0.99 | 0.0001 |
| 11 | Serum creatinine (mg/dL) | 1.17 | 1.19 | 0.18 | 1.14 | 1.22 | 0.18 | 0.7151 |
In patients with severe liver disease, accumulation of toxic metabolites such as bilirubin, ammonia, aromatic amino acid, endotoxin compounds, and benzene products can lead to severe complications and hinder the recovery of the function of hepatocytes and the patient may ultimately progress to hepatic failure.5 In most of the cases in our study, the etiology of liver failure was not known. The TPE helps in maintaining cerebral perfusion pressure and in removing the toxic substances from the patient's plasma, and the FFP, administered as replacement fluid, replenishes the essential substances, such as coagulation factors, albumin, and immunoglobins. This process improves the microenvironment of the liver, which in turn accelerates the regeneration and helps in the functional recovery of hepatocytes.6,7 In the present study, the PT, serum ferritin, and INR levels improved significantly with the TPE (p=0.0001). Our findings were comparable to the research done by Singer et al., who observed that the TPE could correct the bleeding diathesis in acute liver failure patients with the correction of the coagulation profile, when FFP was used as the replacement fluid.8 In the cerebral area of the brain, blood flow and oxygen uptake can be improved with the TPE.9,10 Data on the frequency of the TPE sessions and their duration in these patients is scarce. The MELD score is highly accepted as a measure of the severity of liver failure in patients with irreversible cirrhosis who are awaiting liver transplantation and valid for the estimation of morbidity and mortality.11,12 To calculate the MELD score, the serum creatinine, INR, prothrombin time and bilirubin levels are considered and computed on a continuous scale to avoid the drawbacks of the Child–Pugh score.13 Our study showed that the TPE treatment improves the liver function test and decreases the MELD score in patients with impaired liver function and critical liver failure. It also suggests that the TPE can help support liver functions and gain time for hepatocyte regeneration and thus, may lead to a decrease in the mortality of critical liver disease patients. The survival of patients in our study was 64.44%, which was related to liver function recovery and MELD score improvement. This finding was comparable with research done by Freeman et al., who observed a 55% survival in post-TPE liver failure patients.14 Recently, a large randomized controlled trial (RCT) done by Larsen et al. showed that liver failure patients might have increased survival before liver transplant using high volume plasma exchange, which is corroborative with the findings of the present study.15 We also observed that the post-TPE procedure platelet counts were significantly reduced in all the patients, which is common in all extracorporeal treatments. This platelet loss ranges from 0 to 71% depending upon the patient's clinical diagnosis and the equipment used for the TPE.16 No serious adverse events were observed in the patients during the TPE. The only significant observation was hypocalcemia, which was corrected proactively during the procedure with a slow infusion of 10% calcium gluconate, with frequent arterial blood gas monitoring for ionized calcium. Other advanced methodologies, including continuous veno-venous hemofiltration (CVVHF), which is useful in the removal of medium-sized molecules, ammonia and albumin dialysis with the molecular adsorbent recirculating system (MARS), which can detoxify blood, improve cerebral circulation, and reduce brain edema are also very helpful options for this category of patients, have been variably used and are under active investigation.17 Due to the retrospective nature of this study, patient management could not be blinded, laboratory collection times were not standardized and often incomplete, the TPE was variable in repetition frequency and the fluid balance and vasoactive therapy were not standardized. The number of the patients was comparatively smaller and the total number of TPE procedures performed was variable.
ConclusionBased on the results of our study, we conclude that the TPE improves the liver function tests, coagulation profile and short-term survival in patients with critical liver diseases, despite the continuing poor overall survival. Hence, judicious use of the TPE is recommended as supportive therapy in the performance of liver transplantation in these patients. Randomized control trials are required to further establish the definitive role and effects of the TPE in the treatment of liver failure patients.
Conflicts of interestThe authors declare no conflicts of interest.