The global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to vaccine deployment in record time.1 Very rare cases of thrombotic events associated with thrombocytopenia have only emerged after vaccination of millions of people.2 Acquired Thrombotic Thrombocytopenic Purpura (aTTP) is one of the adverse syndromes that has been progressively reported in the literature. This form of thrombotic microangiopathy (TMA) is associated with presence of autoantibodies against ADAMTS13,3 resulting in formation of platelet thrombi within the microcirculation. Case reports showed that several vaccines against SARS-CoV-2 triggered a de novo or relapse episode of aTTP as summarized by Buetler et al.4 Effective treatment needs to be provided as soon as possible to patients with TTP as it can be a fatal disease. Here, we describe four cases of a newly aTTP diagnosis who had received ChAdOx1 nCov-19, the heterologous recombinant adenovirus combination rAd26 and rAd5 (Gam-COVID-Vac, Sputnik-V) or the inactivated whole virus (BBIBP-CorV, Sinopharm) vaccines.
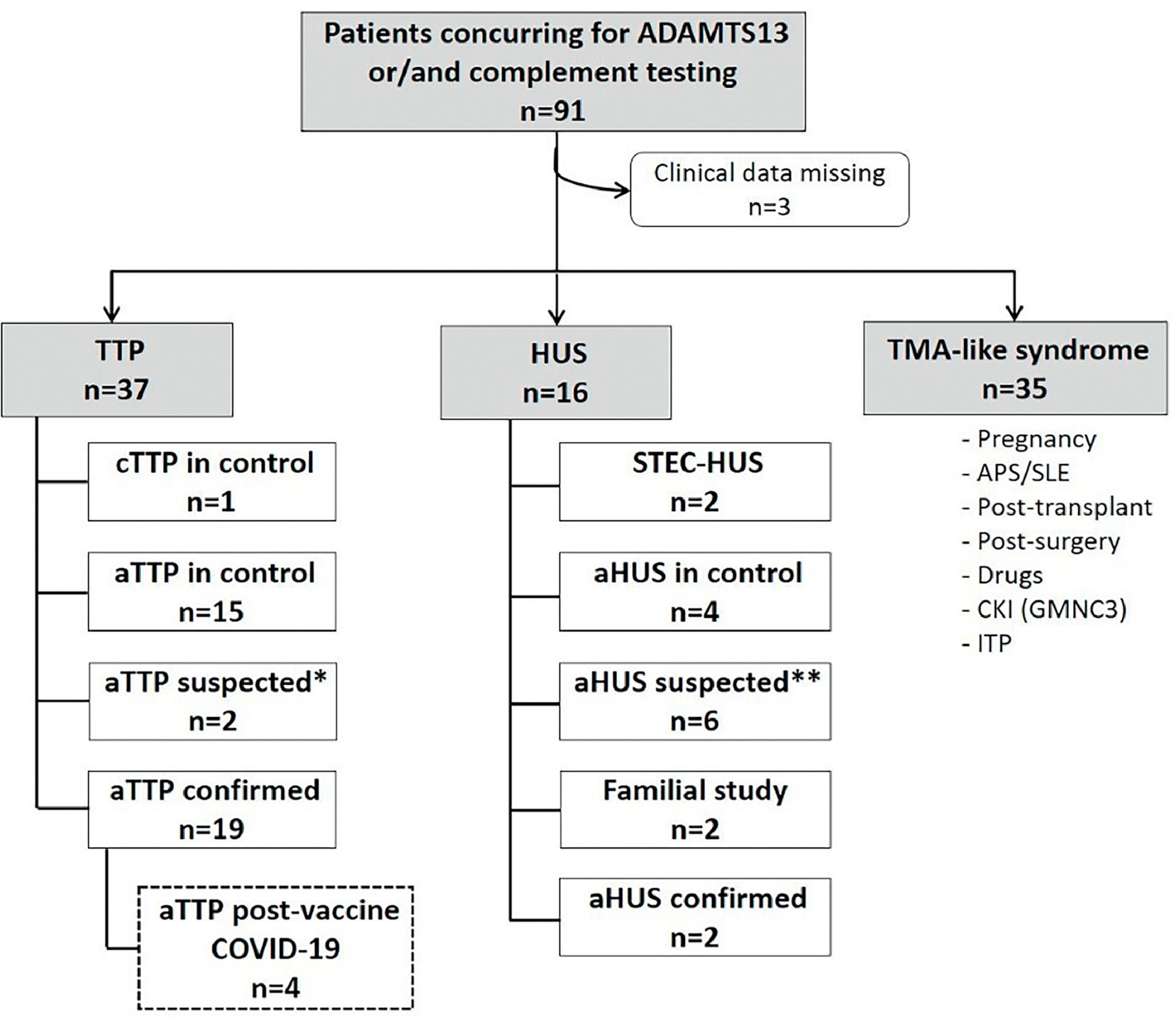
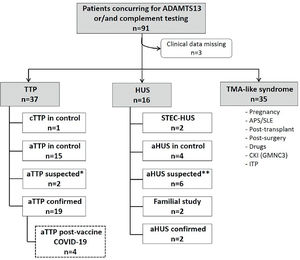
Patients and methodsThe Department of Haemostasis and Thrombosis was the first centre in Argentina to optimize tools for the diagnosis of TMA, and more specifically for TTP and atypical haemolytic uremic syndrome (aHUS). Patients who manifested a non-immune microangiopathic haemolytic anemia (MAHA) (haemoglobin <12 g/dL with schistocytes, elevated LDH), thrombocytopenia (<150 × 109/L platelets count or a decrease of 25 % from baseline) with or without organ injury of variable severity were defined as suspected TMA. As described previously,5 the centre follows a laboratory procedure to confirm the diagnosis (Figure 1). Measurement of ADAMTS13 activity and anti ADAMTS13 IgG antibodies in human plasma was performed using Technozym ELISA (Vienna, Austria). Complement testing was performed when TTP was discarded (ADAMTS13 activity > 10 %) and a diagnostic of aHUS was suspected due to the presence of renal injury. Patients with features of TMA that developed after an identified primary event such as pregnancy, antiphospholipid syndrome (APS) or systemic lupus erythematosus (SLE), surgery, transplant, etc. were categorized separately as TMA-like syndromes (Figure 1).
Flowchart of patients studied in our Department from January to October 2021 with clinical suspicion of TMA. *Patients with clinical symptoms of aTTP whose diagnosis was not confirmed by ADAMTS13 testing due to a lack of adequate samples. **Patients with suspicion of aHUS and negative for complement anomalies. TMA, thrombotic microangiopathy; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; HUS, haemolytic uremic syndrome; aHUS, atypical haemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura; cTTP, congenital thrombotic thrombocytopenic purpura; aTTP, acquired thrombotic thrombocytopenic purpura; APS, antiphospholipid syndrome; SLE, systemic lupus erythematosus; CKI, chronic kidney injury; GMNC3, C3 glomerulonephritis; ITP, immune thrombocytopenic purpura.
Four patients from different healthcare centres in Buenos Aires Province and Federal District (Argentina) are described. Written informed consent for publication was obtained from all patients or patient's family.
Diagnosis, treatment and outcomeFrom January to October 2021, our centre performed ADAMTS13 and/or complement testing in 91 consecutive patients who presented manifestations of TMA (Figure 1). From this cohort, 41 % included patients with TTP, of whom 19 (21 %) were newly diagnosed with aTTP. Two other patients presented with TMA after receiving COVID-19 vaccine. Both showed a rapid response to therapeutic plasma exchange (TPE) but the clinically suspected diagnosis of aTTP could not be confirmed due to the lack of ADAMTS13 testing before treatment. In this report, we described four cases of aTTP related to vaccination against COVID-19 which started at the end of December 2020 in our country. All patients were routinely tested for COVID-19 infection upon hospitalization, and they all tested negative using the PCR method.
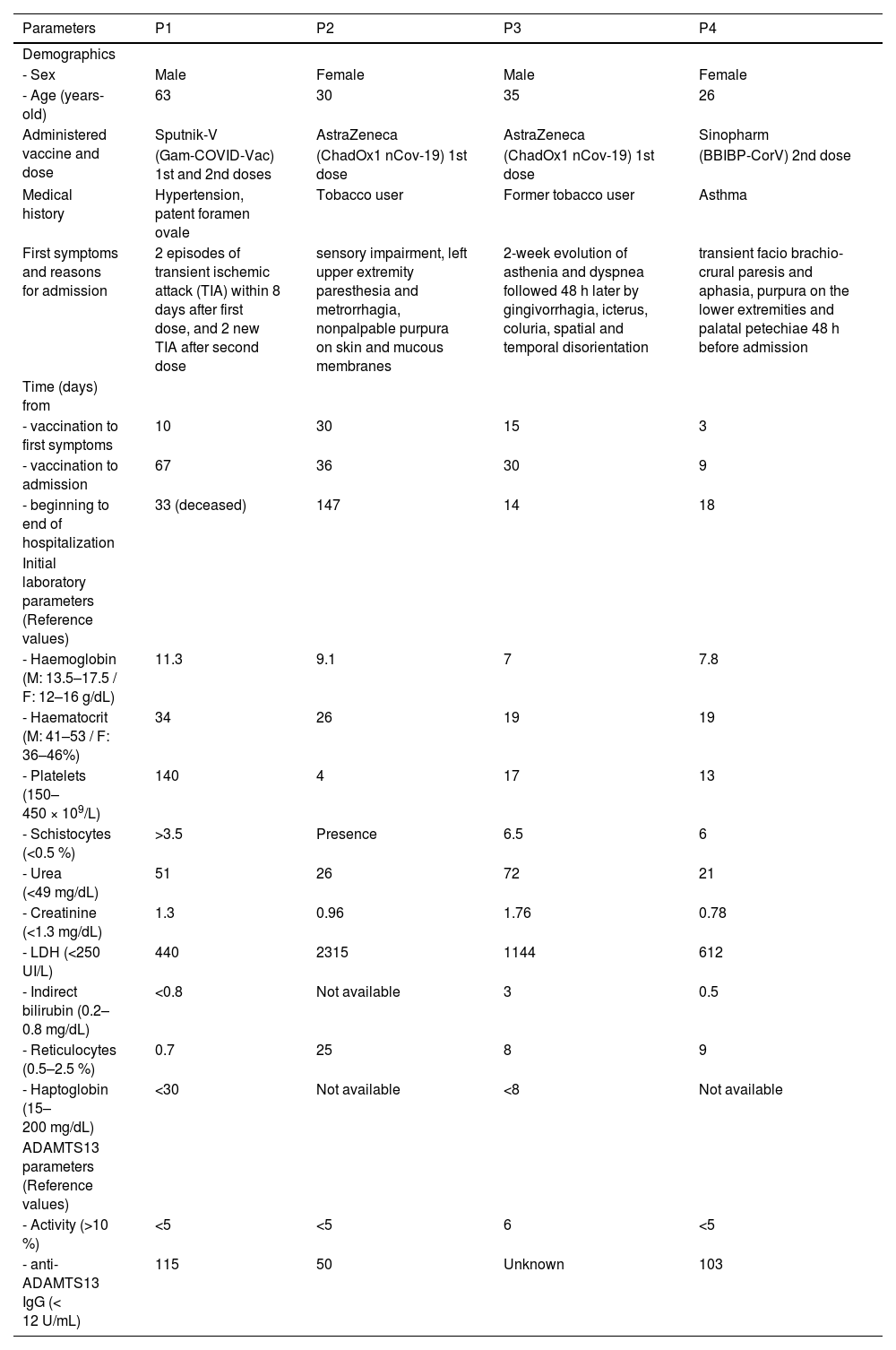
All patients presented with MAHA and thrombocytopenia with organ injury 3 to 30 days after vaccination (Table 1). Patients 1 to 4 (P1-P4) experienced neurological manifestations. P1 and P3 showed variable grades of compromised renal function. All four patients were tested for ADAMTS13 activity during acute phase confirming diagnosis of TTP in three out of four subjects. High levels of anti-ADAMTS13 IgG autoantibodies were reported in P1, P2 and P4 but could not be measured in P3 as the patient's sample was tested at another facility. However, manifestation of neurological symptoms in P3, absence of prior episodes and sustained normalization of ADAMTS13 activity (70 %) during remission strongly supported a diagnosis of aTTP.
Demographic, clinical and laboratory parameters of patients. M: male; F: female.
On the day of admission, since levels of ADAMTS13 activity had confirmed the diagnosis, P1 initiated TPE and methylprednisolone. The patient's clinical course was insidious, characterized by a mild thrombocytopenia that gradually deepened. At day 11 after admission, multi-territory ischemic stroke was detected. Considering the refractoriness of the response, the patient started to receive rituximab and bortezomib. Patient showed no response to new methylprednisolone pulse in association with TPE every 12 h. Disease progression and deterioration were observed with the patient suffering another stroke and worsening of blood parameters (Hb: 6.6 g/dL), platelet count (15 × 109/L) and renal function (Ur: 173 mg/dL). The patient died within 24 h of acute kidney injury and respiratory insufficiency on day 33 of hospitalization.
All three younger patients diagnosed with aTTP exhibited serious neurological features; nevertheless, they all presented favourable outcomes after treatment. P2 received TPE and methylprednisolone pulse. Rapid recovery was observed with normalization of hematologic parameters. P3 experienced the same outcomes after receiving TPE conjugated with corticosteroids. Upon admission, P4 received methylprednisolone pulse followed by administration of 60 mg/day prednisone for two weeks. Upon completion of the TPE sessions and continuation of plasma infusion for 48 h, P4 recovered from anaemia and thrombocytopenia. One week after discharge, P4 began a plan to taper off prednisone.
DiscussionThe delay in P1 admission could be explained by preventive and mandatory isolation imposed by the government largely on the population over 60, and the fact that health facilities at the time were obliged to concentrate medical care almost exclusively on COVID-19. Neurological and renal manifestations observed in P1 illustrated data from literature showing higher prevalence of affected organs and onset signs in patients aged 60–65.6,7
Between December 29 2020, and October 31 2021, 48,588,926 doses of SARS-CoV-2 vaccines were administered in Argentina, 96 % of which were Gam-COVID-Vac (14,867,556), ChAdOx1 nCov-19 (16,448,334) and BBIBP-CorV (15,243,533). 59 % of the total (28,640,295) were applied in Buenos Aires Province and the Federal District (https://www.argentina.gob.ar/coronavirus/vacuna/aplicadas). Although previous observations showed a correlation between aTTP and administration of either mRNA-based (BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna)) or adenoviral vector-based vaccines ChAdOx1 nCov-19 and Ad26.COV2-S (Janssen),4 it is to our knowledge the first report of a newly diagnosed aTTP associated with Gam-COVID-Vac or BBIBP-CorV. While the novel disorder “vaccine-induced immune thrombotic thrombocytopenia” (VITT) was mainly observed after vaccination based on adenoviral vector (AstraZeneca and Janssen), it seems immune TTP could be triggered by any type of COVID-19 vaccine platform. Although the period between vaccine administration and first symptoms in P4 may be too short to show adverse events as observed previously,8 no other trigger was identified that could explain the onset of TMA episode described here.
In our cohort of newly diagnosed aTTP within a 10-month period, 21 % of the cases were associated with Sars-CoV-2 vaccination. As similar cases were not identified in healthcare centres outside Buenos Aires, we cannot deny the presence of referral bias in our report. However, during the period of our study, no other case of aTTP after immunization was reported in our national system of vigilance for adverse events. From January 2017 to July 2022, our department diagnosed an average of 17 new aTTP episodes per year (0.377 per million), representing an increase in the annual incidence of aTTP previously described in our region (0.2 per million).5 Here, we reported 19 newly aTTP cases diagnosed between January and October 2021, which is slightly above this average. However, it seems that the estimated incidence of aTTP after immunization considering our vaccinated population in the same period (0.139 per million) is not higher than the annual incidence of aTTP in Argentina.
Two primary hypotheses were proposed to explain the pathophysiology of aTTP induced by vaccines. One mechanism under consideration could be linked to molecular mimicry. Immune cross-reactivity between vaccine components and self-proteins, leading to the formation of autoantibodies anti-ADAMTS13, has previously been suggested as a potential process following Influenza/H1N1,9-11 pneumococcal,12 or rabies 13 vaccination. However, the temporal relationship between vaccine administration and the onset of the first symptoms in some reported cases, both in our study and in the literature, is unlikely to support this hypothesis. Since the publication of the initial case reports, larger multicenter or single-center cohort studies have provided data on the incidence of de novo or relapsed aTTP in their vaccinated populations.8,14,15 They showed that the incidence of de novo or relapse aTTP did not increased with the administration of the COVID-19 vaccine, with rates of 0.195 per million in the United States 8 and 0.118 per million in France, considering only cases occurring within 30 days of vaccination.14 Additionally, recent data from the literature showed no cross-reactivity between autoantibodies to ADAMTS13 and the SARS-CoV-2 S1 Spike protein binding.16 The alternative hypothesis is that patients already had an undetected ADAMTS13 activity deficiency at the time of vaccination and the process of immunization acted as a trigger for the onset of the episode. This could more likely explain why some patients experienced TMA manifestations shortly after receiving the vaccine. While no further studies support either hypothesis, the significance of monitoring the vaccine response in patients with a history of acquired or congenital TTP has been emphasized.8,14
ConclusionsThis is the first report of aTTP cases after the administration of Gam-COVID-Vac or BBIBP-CorV. Both vaccines are in addition to those already described in the literature to be precipitating events in anti-ADAMTS13 autoimmunity. Although the prevalence seems very low in the context of rare diseases, knowledge of such possible events should be spread widely in order to diagnose patients accurately and treat them rapidly.