Exacerbated inflammation and coagulation are a hallmark of COVID-19 severity. Extracellular vesicles (EVs) are intercellular transmitters involved in inflammatory conditions, which are capable of triggering prothrombotic mechanisms. Since the release of EVs is potentially associated with COVID-19-induced coagulopathy, the aim of this study was to evaluate changes in inflammation- and hypercoagulability-related EVs during the first month after symptom onset and to determine whether they are associated with disease severity. Blood samples of patients with mild or severe forms of the disease were collected on three occasions: in the second, third and fourth weeks after symptom onset for the quantification by flow cytometry of CD41A (platelet glycoprotein IIb/IIIa), CD162 (PSGL-1), CD31 (PECAM-1) and CD142 cells (tissue factor). Analysis of variance (ANOVA) with repeated measures, Kruskal-Wallis and correlation tests were used. Eighty-five patients were enrolled, 71% of whom had mild disease. Seventeen uninfected individuals served as controls. Compared to controls, both mild and severe COVID-19 were associated with higher EV-CD31+, EV-CD41+ and EV-CD142+ levels. All EV levels were higher in severe than in mild COVID-19 only after the third week from symptom onset, as opposed to C-reactive protein and D-dimer levels, which were higher in severe than in mild COVID-19 earlier during disease progression. EV levels were also associated with C-reactive protein and D-dimer levels only after the third week of symptoms. In conclusion, EVs expressing CD41A, CD31, TF, and CD162 appear as late markers of COVID-19 severity. This finding may contribute to the understanding of the pathogenesis of acute and possibly long COVID-19.
Severe forms of coronavirus disease 2019 (COVID-19) are associated with exacerbated inflammation, involving cytokine release, endothelial dysfunction, and platelet activation, leading to a state of hypercoagulability. 1 Although COVID-19-associated coagulopathy (CAC) is similar to disseminated intravascular coagulation (DIC) and sepsis-induced coagulopathy (SIC), 2 some unique features are seen, such as elevated plasmas fibrinogen levels and increased factor VIII activity. 1,3
Substantial crosstalk between inflammation and coagulation in COVID-19 is observed by a variety of clinical and laboratory findings. These include elevated levels of factor VIII, fibrinogen, von Willebrand factor, and D-dimer; the presence of neutrophil extracellular traps (NETs); platelet activation; and the presence of circulating prothrombotic extracellular vesicles (EVs). 2-4
EVs are small circulating vesicles derived from cell membranes and released by activated cells under both physiological and pathological conditions. They act as messengers between cells and are capable of encapsulating and transferring proteins, RNA, miRNAs, carbohydrates, DNA and lipids. 5 In inflammatory conditions, cardiovascular diseases and cancer, EVs are released by various cell types, including platelets, erythrocytes, leukocytes (neutrophils, monocytes, and T and B lymphocytes), dendritic cells, endothelial cells, hepatocytes and tumor cells. 6 EVs can also modulate immune cell activity, exerting an inflammatory role 7 or even inducing an immune surveillance capacity. 8
A number of markers for cells and proteins involved in the inflammatory response have been described, such as CD41A, CD162, CD31. CD41A, platelet surface glycoprotein receptor IIb/IIIa, is derived from activated platelets, 9,10 CD162 (P-selectin-binding glycoprotein [PSGL-1]) plays a critical role in leukocyte trafficking during inflammation by being responsible for the interaction between leukocytes and activated platelets or endothelial cells that express selectins. 11 CD31, or platelet-endothelial cell adhesion molecule-1 (PECAM-1), is an endothelial antigen that serves as a marker of platelet-endothelial interactions. 9
In addition to inflammation, one of the most important functions of EVs is the promotion of blood coagulation. This is due to the presence of large amounts of negatively charged phospholipids exposed on the surface of the plasma membrane of EVs, such as phosphatidylserine, 12 and to the expression of active tissue factor (TF), also known as CD142 on their surface. TF is responsible for the coagulation process initiated after endothelial injury, which is the main trigger of the extrinsic pathway. 13,14
From a clinical perspective, EVs may serve as markers for critical forms of COVID-19. For this, it is necessary to determine whether EV levels can identify, at an early stage in the progression of COVID-19, cases at higher risk of adverse outcomes. Therefore, the aim of this study is to evaluate changes in inflammation- and hypercoagulability-related EVs during the first month of SARS-CoV-2 infection. It was also determined whether EVs are associated with disease severity at different timepoints during the clinical course of the disease.
MethodsStudy participantsThis is a longitudinal, prospective, single-center observational study (NCT04466670) that started in July 2020, after approval by the Hospital das Clinicas Committee of Ethics in Research (CAPPesq-HCFMUSP) and by the National Commission of Ethics in Research (CONEP, CAAE 33788820.9.0000.0068). Informed consent was obtained from all the participants or their legal representatives. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
The study cohort included individuals aged 18 years or older, who were diagnosed with COVID-19 by specific laboratory criteria (molecular RT-PCR-SARS-CoV-2 or rapid antigen test). These individuals were divided into two groups according to the severity of the disease. Patients with severe COVID-19 were those admitted to the intensive care unit (ICU) of the Hospital das Clinicas of the University of Sao Paulo Medical School with acute respiratory failure and PaO2/FiO2 <200 mmHg. Patients with mild COVID-19 were those who were able to be treated in isolation at home. Patients were recruited from July 2020 to November 2020. Exclusion criteria were the use of an anticoagulant at full dose at the time of screening, history of home oxygen dependence, pregnancy, risk of death within 24 h, use of palliative care and bacterial endocarditis. For participants, demographic and clinical data, laboratory parameters and radiological findings performed in patient care routine were recorded. Patients’ blood samples were collected in tubes containing 3.2% sodium citrate on three occasions: in the second (6th to 10th day), third (16th to 20th day) and fourth weeks after the onset of symptoms (21st to 25th day).
Seventeen controls were tested to determine EV levels in uninfected individuals. The control group consisted of healthy individuals, the majority of whom were women (n = 12, 71%), with a median age of 39 years. The remainder reported comorbidities such as hypertension (n = 2), obesity (n = 2) and diabetes (n = 1), but without personal history of thrombosis, acute infectious or inflammatory condition, or pregnancy.
Laboratory proceduresTo obtain the EVs, blood samples were centrifuged at 3000 × g for 20 min at room temperature to remove platelets and debris. A residual platelet count was then performed on a sample of the resulting platelet-poor plasma (PPP) to ensure a count of <10 × 103/μL. The PPP was subsequently washed with Dulbecco's phosphate buffered saline (DPBS - Sigma-Aldrich) without calcium or magnesium, followed by centrifugation at 20,000 × g for 30 min at 4 °C. The resulting pellet was resuspended in 100 μL of DPBS and stored at −80 °C until use.
EVs were quantified by flow cytometry on the Beckman Coulter CytoFLEX. Rosetta Calibration beads (Exometry) for the location of the positive regions for EVs were used to standardize the analysis by flow cytometry. 15,16 A gate corresponding to 300 nm was selected to identify EVs based on their size. After calibration, samples were acquired by the cytometer with the following capture configuration: 1000 events for 120 s and flow rate set to ‘slow’ at 10 µL/min. EVs were first detected as lactadherin (Haematologic Technologies Inc, US) and calcein (Thermo Fisher, US) double-positive events, to distinguish them from apoptotic bodies. Next, within the double labeling events, the positivity of CD41A (BioLegend Cat# 303716, RRID:AB_10897646), CD162 (BioLegend Cat# 328812, RRID:AB_2750485), CD31 (BioLegend Cat# 303106, RRID:AB_314332) and CD142 (Thermo Fisher Scientific Cat# 12-1429-42, RRID:AB_2572581) were selected.
Statistical analysisDescriptive analysis was performed using frequency tables for categorical variables. Numerical variables are expressed as the mean and standard deviation (for normally distributed variables) or median and interquartile range (for non-normally distributed). First, EV levels were compared between controls, mild and severe COVID-19 by the Kruskal-Wallis test. Next, changes in EV marker levels in the second, third and fourth weeks of symptoms were evaluated by analysis of variance (ANOVA) with repeated measures. Each marker was independently evaluated. The Kruskal-Wallis test was also used to compare the laboratory parameters between mild, severe non-fatal and fatal COVID-19 patients at the three timepoints studied.
Finally, the Spearman correlation test was used to assess the correlation between EV marker levels, acute phase inflammatory biomarkers (C-reactive protein - CRP) and hypercoagulability (D-dimer). Statistical analyses were performed with GraphPad Prism software version 8.0 for Windows (GraphPad Software, San Diego, CA, USA).
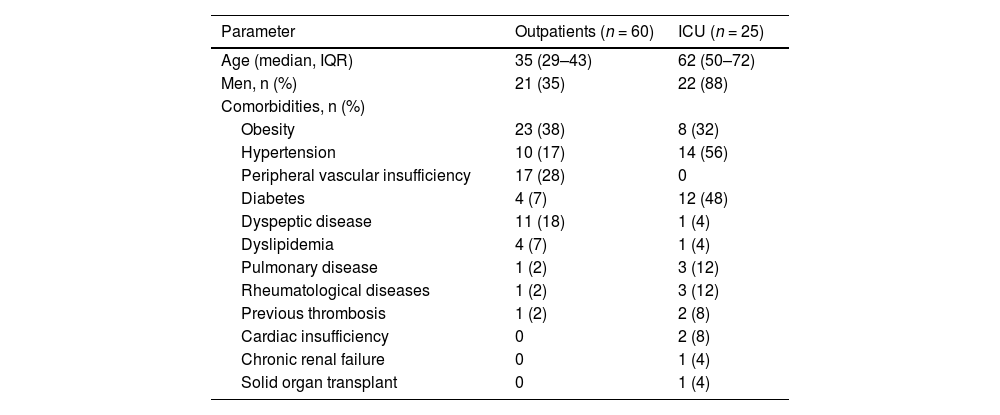
ResultsA total of 85 patients were enrolled in the study. The flow chart of the study is shown in Figure 1. The demographic and clinical characteristics of the patients are shown in Table 1. Sixty patients (71%) had mild COVID-19 (outpatients) and twenty-five patients (29%) had severe disease and were hospitalized in the ICU. The majority of the outpatients were women (n = 39; 65%) with a median age of 35 years [interquartile range (IQR): 29–43], whereas the majority of the ICU patients were men (n = 22; 88%) with a median age of 62 years (IQR: 50–72). Comorbidities were more frequently reported by ICU patients, the most common being hypertension (56%), diabetes (48%) and obesity (32%).
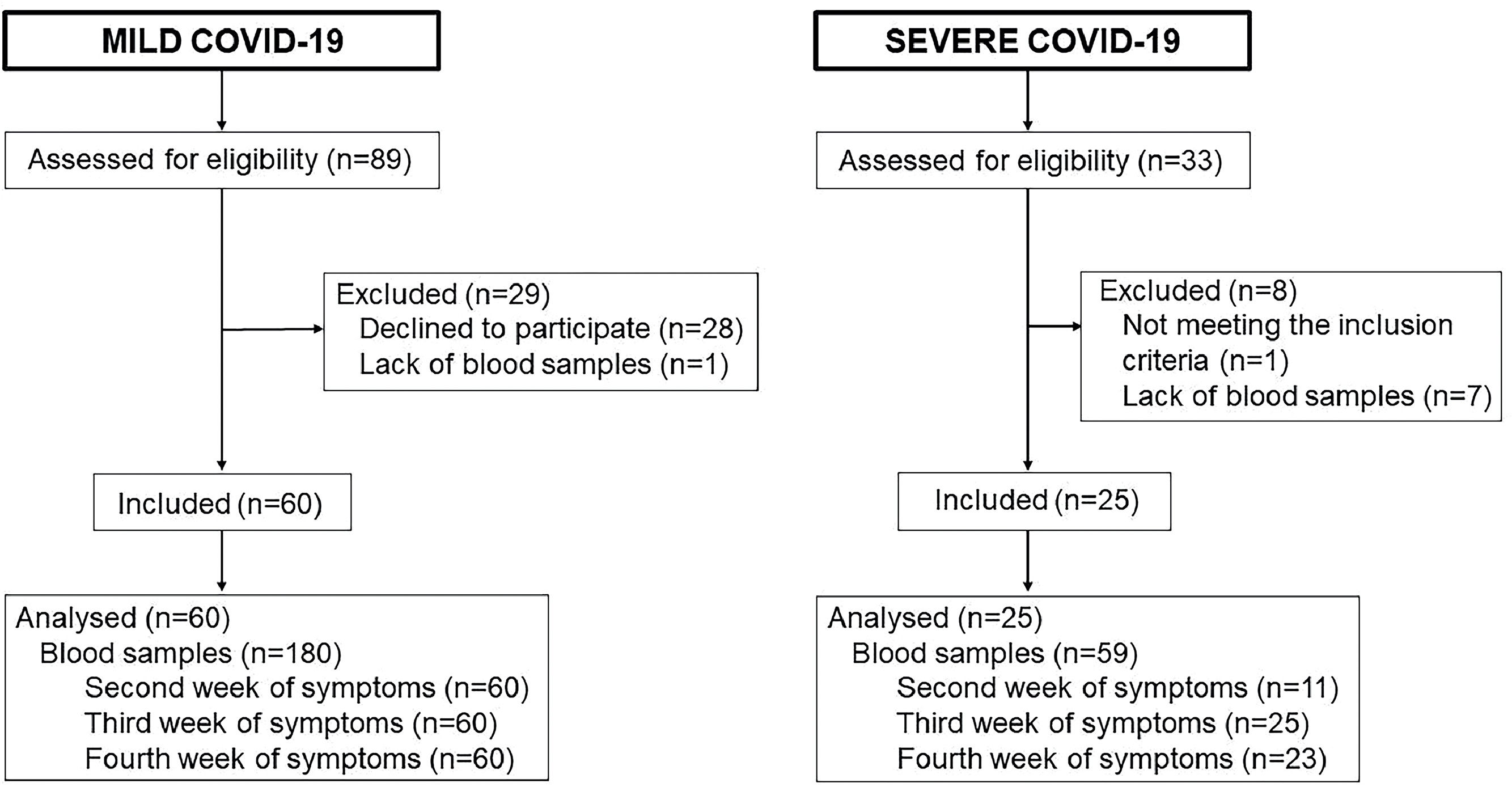
Flowchart of patient selection and reasons for exclusion. The flowchart demonstrates participant's selection, reasons for exclusion and final sample size. A total of 89 outpatients were initially recruited but 28 were excluded for declining to participate and one for failure of blood collection, resulting in 60 outpatients included in the study. A total of 33 ICU patients were recruited and, after eight being excluded (for lack of inclusion criteria or blood sample), 25 were included in the study. For both groups blood samples were collected on three occasions: second week of symptoms (n = 71), third week of symptoms (n = 85) and fourth week of symptoms (n = 83), totaling 239 samples.
Demographic and clinical characteristics of patients with COVID-19.
Abbreviations: n: absolute number of patients; IQR: interquartile range; ICU: intensive care unit. Pulmonary disease includes asthma and chronic obstructive pulmonary disease. Previous thrombosis includes arterial and venous thrombosis.
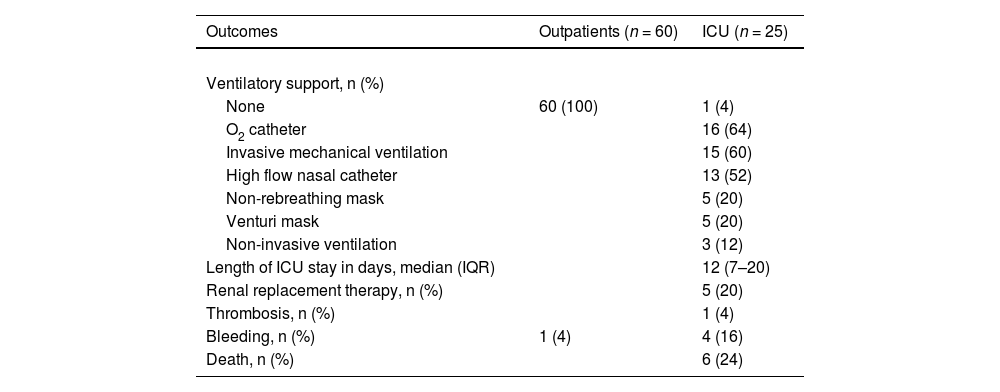
Table 2 shows the progression and outcomes of COVID-19 in mild and severe patients. During the course of the disease, 60% of the severely ill patients required invasive mechanical ventilation, 20% required renal replacement therapy and 24% died. The median ICU stay was 12 days (IQR: 7–20). Mild patients had no adverse outcomes during the course of the infection.
Progression and outcomes of COVID-19 in outpatients and intensive care unit (ICU) patients.
Abbreviations: n: absolute number of patients; IQR: interquartile range (25 and 75 percentiles). One thrombotic event (mesenteric thrombosis) occurred in the fourth week after COVID-19 symptom onset in an ICU patient. Two minor bleeding events (epistaxis and bleeding at puncture site) occurred in the third week and two (epistaxis and menorrhagia) in the fourth week after the onset of symptoms in ICU patients. Only one bleeding event was reported in outpatients, an abnormal uterine bleeding at week 4 after symptom onset.
All ICU patients received antithrombotic prophylaxis, either enoxaparin or unfractionated heparin, while only one outpatient received this treatment. One thrombotic event (venous mesenteric thrombosis) occurred in the fourth week after COVID-19 symptom onset in an ICU patient. Two minor bleeding events (epistaxis and bleeding at puncture site) occurred in the third week and two (epistaxis and menorrhagia) in the fourth week after the onset of symptoms in ICU patients. Only one bleeding event was reported in outpatients, an abnormal uterine bleeding in week 4 after symptom onset.
Levels of extracellular vesicles at baseline in COVID-19 patients compared to controlsEV-CD31+, EV-CD41+ and EV-CD142+ levels were higher in both mild and severe COVID-19 than in controls however EV-CD162+ levels did not differ between COVID-19 patients and controls. Median EV-CD162+ levels were 0.70 events/µL (IQR: 0.35–1.25) in controls, 0.32 events/µL (IQR: 0.11–0.75; p-value = 0.10) in mild COVID-19 and 1.30 events/µL (IQR: 0.30–2.50; p-value = 0.99) in severe COVID-19 at week 2.
Median EV-CD31+ levels were 1.42 events/µL (IQR: 0.72–2.14) in controls, 8.40 events/µL (IQR: 3.74 - 13.79; p-value = 0.0004) in mild COVID-19 and 9.65 events/µL (IQR: 2.60–19.70; p-value = 0.004) in severe COVID-19 at week 2.
Median EV-CD41A+ levels were 1.75 (IQR: 1.05–3.83) in controls, 5.30 events/µL (IQR: 1.55–11.23; p-value = 0.02) in mild COVID-19 and 17.25 events/µL (IQR: 4.25–23.60; p-value = 0.004) in severe COVID-19 at week 2.
Median EV-CD142+ levels were 2.35 events/µL (IQR: 1.40–5.83) in controls, 8.45 events/µL (IQR: 4.51–16.06; p-value = 0.005) in mild and 13.90 events/µL (IQR: 6.40–21.55; p-value = 0.003) in severe COVID-19 patients in the second week of symptoms.
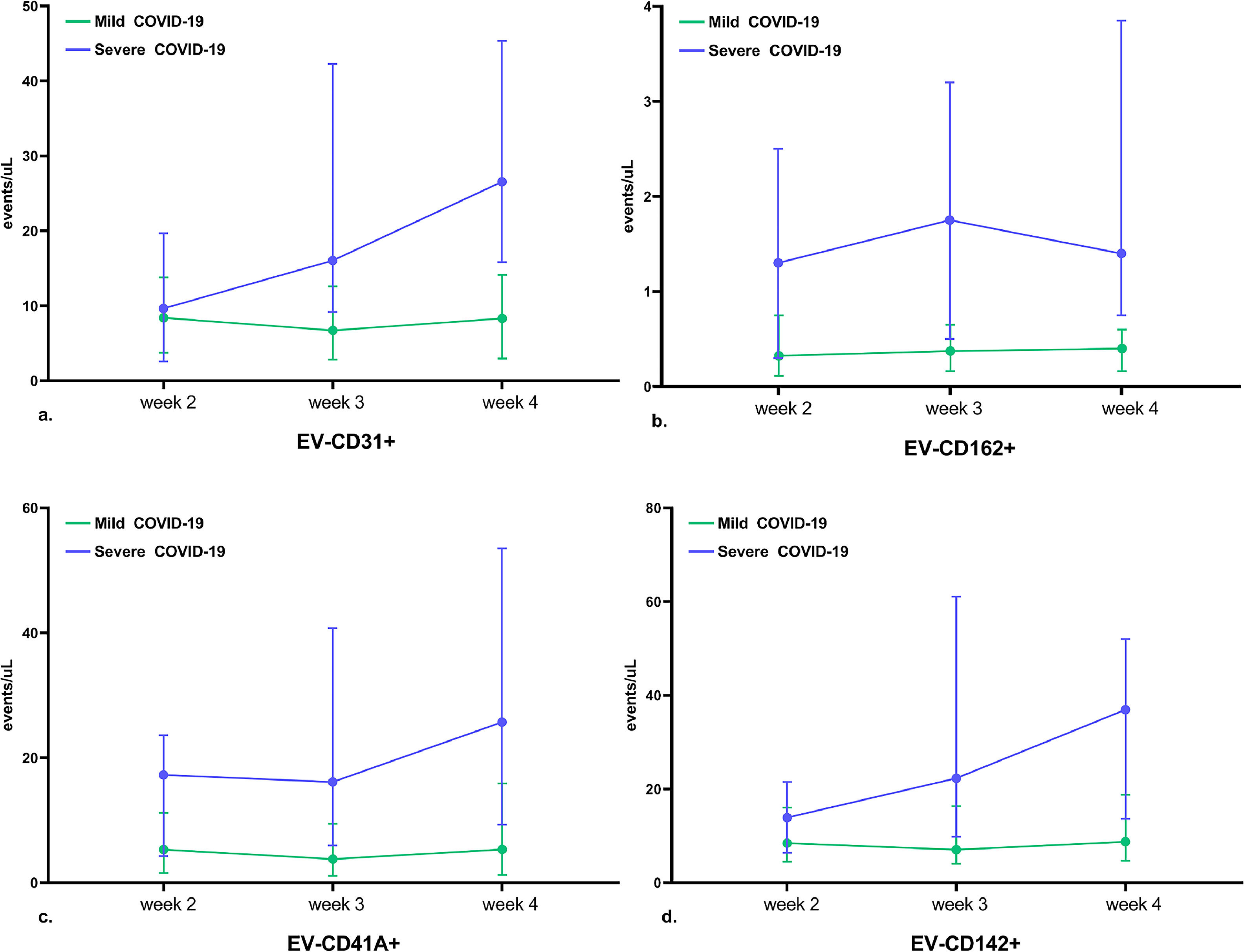
Circulating extracellular vesicle levels during the course of mild and severe COVID-19Figure 2 illustrates the changes in EV-CD31+, EV-CD162+, EV-CD41+ and EV-CD142+ levels during the first month of mild and severe COVID-19 progression. There was no significant change in the levels of EVs either in the mild cases or in the severe COVID-19 cases.
Levels of circulating extracellular vesicles (EVs) during the course of COVID-19. This figure illustrates changes in the levels of circulating EVs during mild (blue) and severe (green) COVID-19. a. EV-CD31+ levels; b. EV-CD162+ levels; c. EV-CD41+ levels; and d. EV-CD142+ levels. There was no significant change in the levels of EV during COVID-19, either in the mild cases or in the severe cases. Points and bar errors estimate the median levels and interquartile ranges of EV markers in the second (6th to 10th day), third (16th to 20th day) and fourth (21st to 25th day) weeks after the onset of COVID-19 symptoms. All p-values were greater than 0.05. P-values were calculated using analysis of variance (ANOVA) with repeated measures.
In outpatients with mild COVID-19, EV levels did not change significantly over the course of the disease. In patients with severe COVID-19, the levels of all EVs remained high during the first month of the disease and did not change significantly over time.
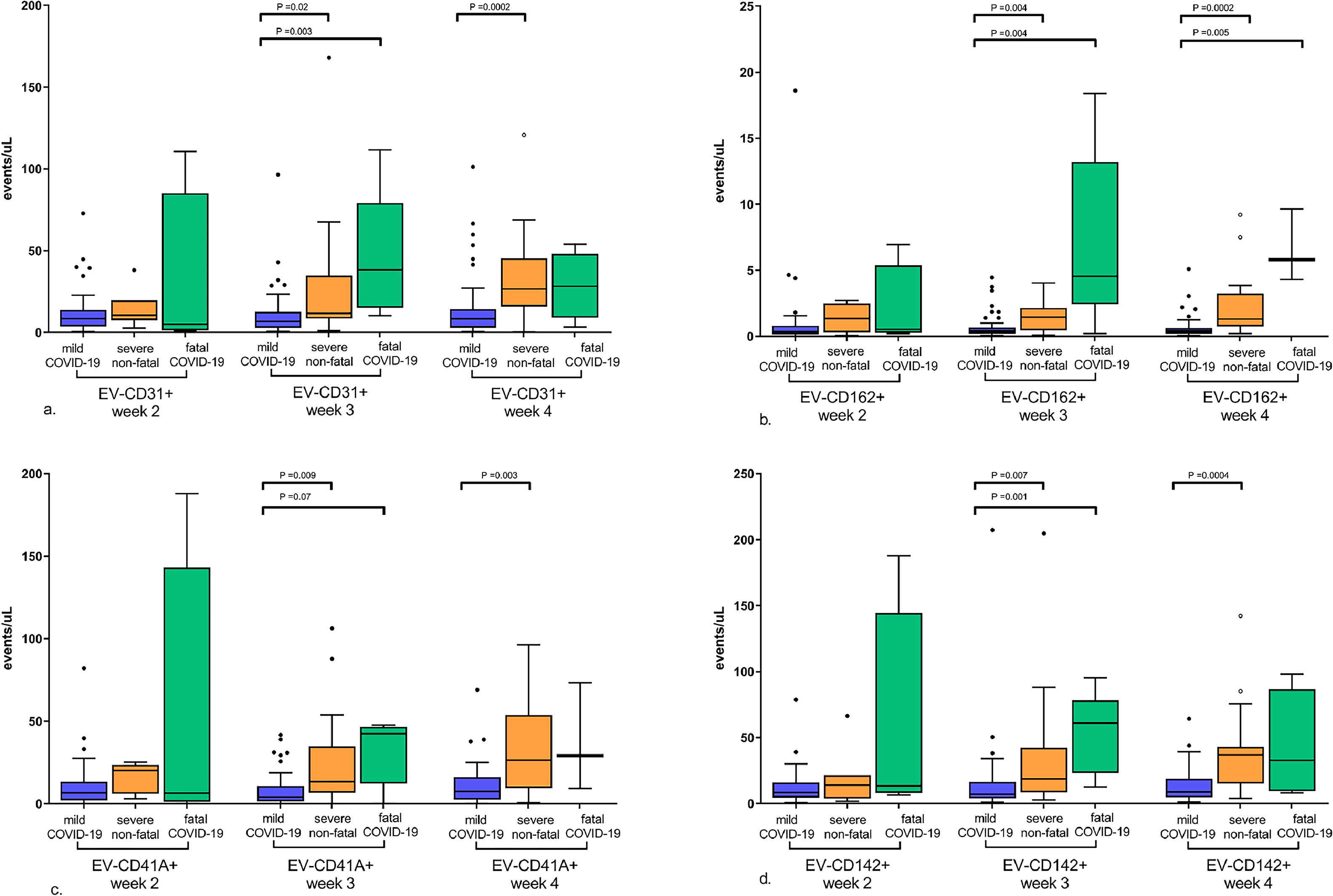
Association of extracellular vesicle markers and COVID-19 severityFigure 3 illustrates the differences in the EV levels between mild, severe non-fatal and fatal COVID-19 during the course of the disease. As shown in the figure, in the second week after symptom onset, the levels of EV-CD31+, EV-CD162+, EV-CD41+ and EV-CD142+ were similar between patients with mild and severe or fatal COVID-19. Levels of EV-CD31+, EV-CD162+, EV-CD41+ and EV-CD142+ in severe or fatal COVID-19 became higher than those in mild COVID-19 only in the third week after symptom onset.
Circulating extracellular vesicle (EV) levels during COVID-19 progression according to disease severity. This figure shows the differences in the levels of EVs between the mild, severe non-fatal and fatal COVID-19 during the progression of the disease. At the onset of symptoms there were no significant differences in EVs levels. However, from week 3, the levels of all EVs were higher in severe and fatal COVID-19 compared to mild disease. a. EV-CD31+ levels; b. EV-CD162+ levels; c. EV-CD41+ levels; and d. EV-CD142+ levels. The graphs represent median and interquartile range of EV markers levels in the second (6th to 10th day), third (16th to 20th day), and fourth (21st to 25th day) weeks after the onset of COVID-19 symptoms. Significant p-values are shown in the graphs. P-values were calculated by the Kruskal-Wallis test.
In addition, there was a trend toward higher levels of EV-CD31+, EV-CD162+, and EV-CD142+ in fatal COVID-19 compared to severe non-fatal COVID-19 after week 3, but the differences were not statistically significant.
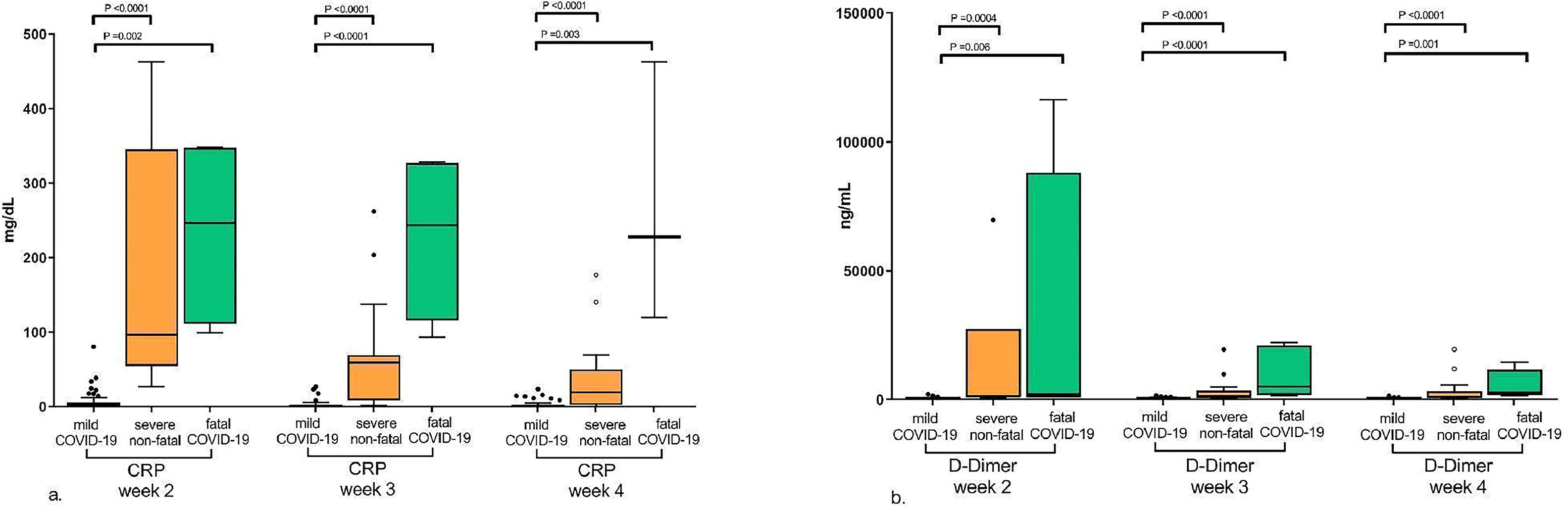
Association between extracellular vesicle levels and coagulation and inflammation markersFigure 4 illustrates the differences in the levels of the coagulation and inflammation markers, D-Dimer and CRP, respectively, between mild, severe non-fatal and fatal COVID-19 during the course of the disease. As shown in the figure, since the second week after symptom onset the levels of CRP and D-dimer were significantly higher in severe and fatal COVID-19 compared to mild disease showing that these are early markers of disease severity and can be observed from the first weeks of symptoms.
Levels of C-reactive protein (CRP) and D-Dimer during COVID-19 progression according to disease severity. This figure shows the differences in the CRP and D-Dimer levels between mild, severe non-fatal and fatal COVID-19 during the progression of the disease. The levels of CRP and D-dimer were higher in severe and fatal COVID-19 compared to mild disease from the second week after the onset of symptoms. a. CRP levels; b. D-Dimer levels. The graphs represent median and interquartile range of EV marker levels in the second, third and fourth weeks after the onset of COVID-19 symptoms. Significant p-values are shown in the graphs. P-values were calculated using the Kruskal-Wallis test.
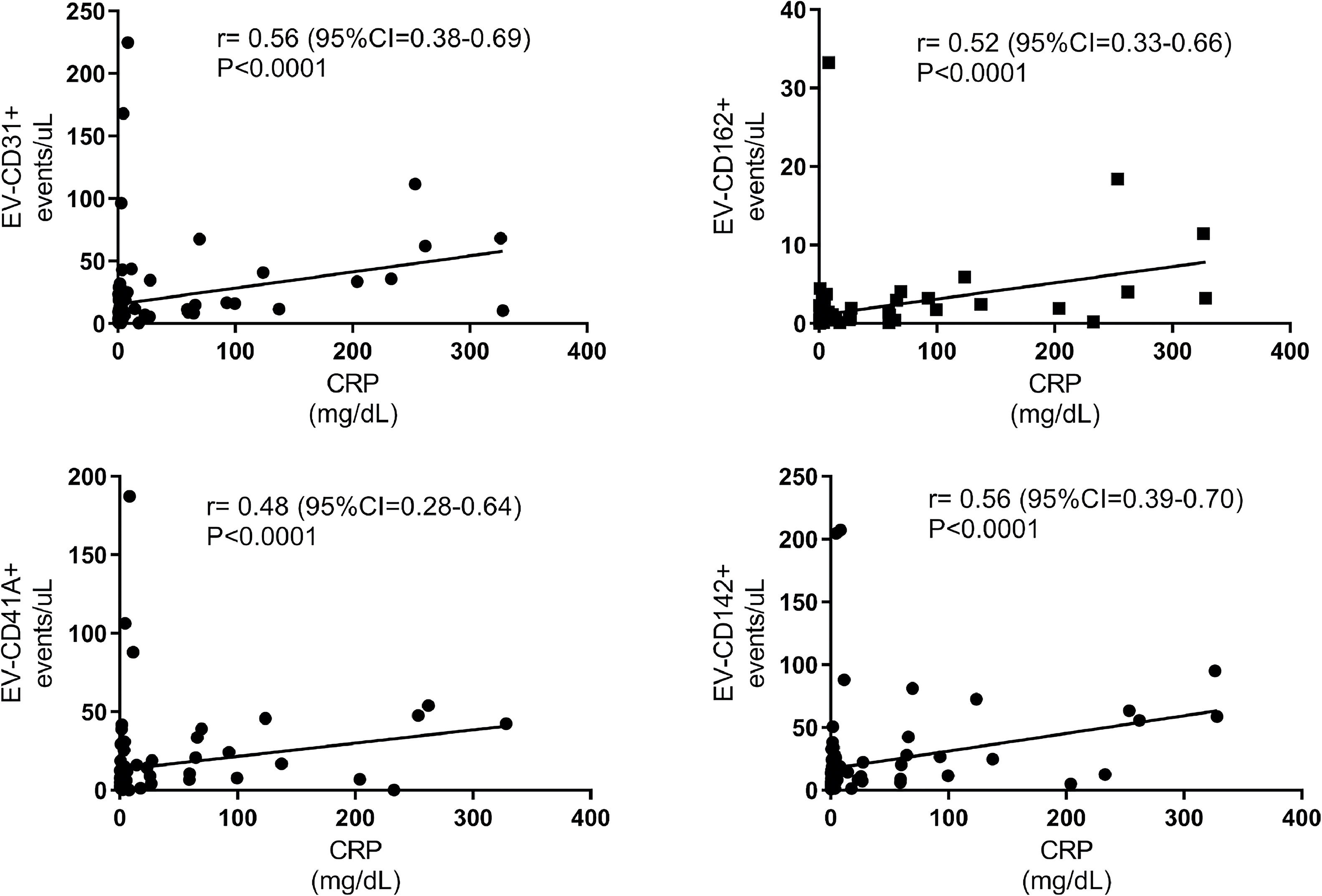
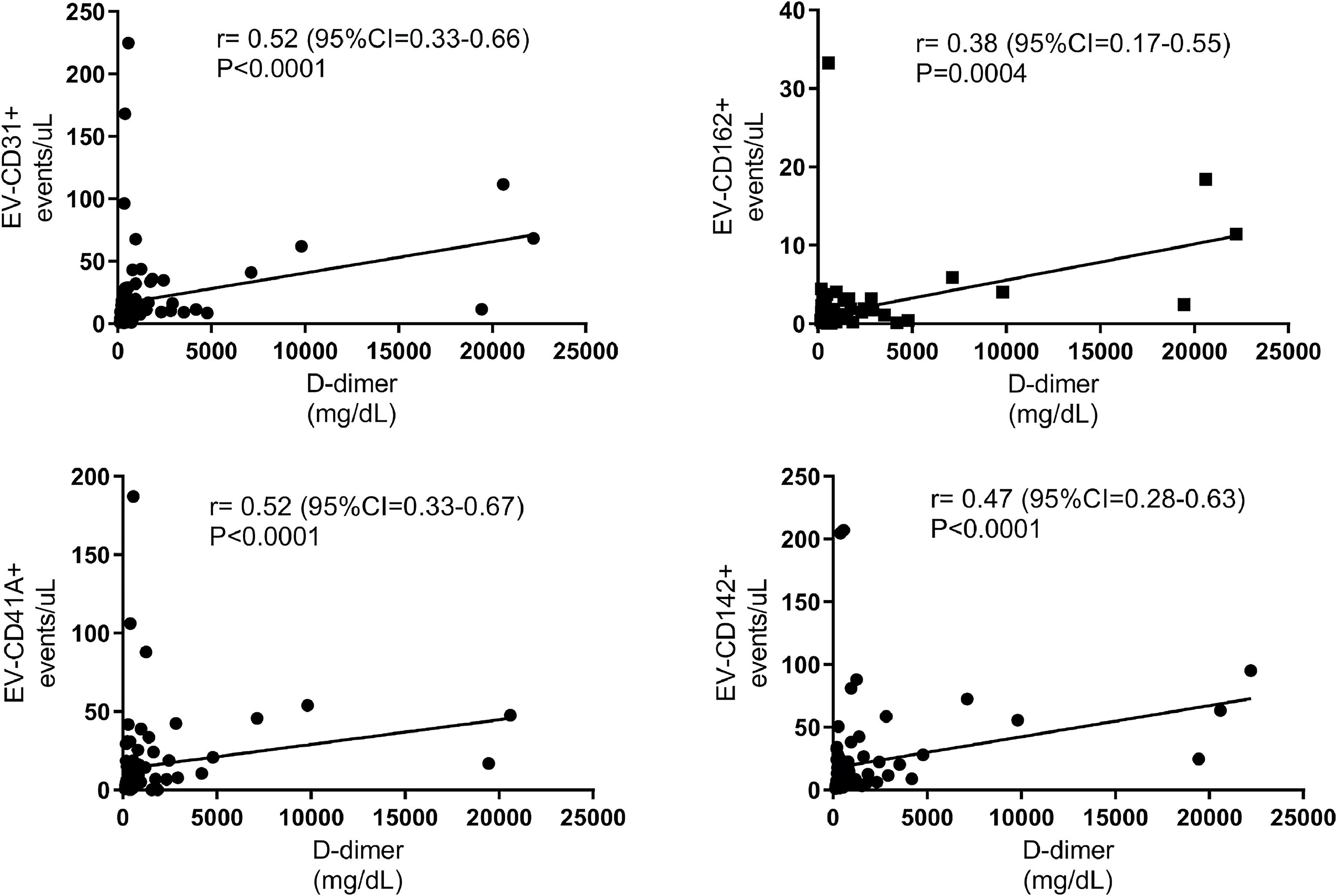
In the second week of symptoms, there was no correlation between CRP and D-dimer levels with EV-CD31+, EV-CD162+, EV-CD41+, and EV-CD142+ levels. However, after the third week of COVID-19 symptoms, a strong positive correlation was observed of CRP and D-dimer with EVs. Figures 5 and 6 illustrate the correlation of CRP and D-dimer levels with EV-VD31+, EV-CD162+, EV-CD41A+ and EV-CD142+ levels in week 3 after the onset of COVID-19 symptoms.
Correlation between C-reactive protein (CRP) and extracellular vesicle (EV) levels in the third week of COVID-19 symptoms. This figure illustrates the significant positive correlation between CRP and the levels of EV-CD31+, EV-CD162+, EV-CD41A+, and EV-CD142+ in the third week after the onset of COVID-19 symptoms.
Correlation between D-dimer and extracellular vesicle (EV) levels in the third week of COVID-19 symptoms. This figure illustrates the significant positive correlation between D-dimer and the levels of EV-CD31+, EV-CD162+, EV-CD41A+, and EV-CD142+ in the third week after the onset of COVID-19 symptoms.
As shown in Figures 5 and 6, EV-CD31+, EV-CD162+, EV-CD41+, and EV-CD142+ levels correlated positively with D-dimer and CRP levels in the third and fourth weeks of symptoms.
DiscussionIn this study, it was observed that both mild and severe COVID-19 are associated with increased levels of EV-expressing platelet antigens (CD41A), endothelial antigens (CD31) and TF (CD142). However, there is no substantial difference in EV levels between mild and severe cases of COVID-19 in the early stages of the disease, as opposed to CRP and D-dimer, which are higher in severe COVID-19 than in mild disease early in the clinical course of the disease. It is only after the third week of symptoms that severe COVID-19 shows EV levels significantly higher than mild COVID-19. EV levels were also associated with inflammatory and coagulation markers only after the third week of symptoms. Therefore, EV-expressing CD41A, CD31, TF and CD162 (PSGL-1), a platelet/leukocyte-endothelium adhesion antigen, appear as late markers of COVID-19 severity.
The pathogenesis of severe COVID-19 is known to be associated with endothelial infection and dysfunction, 17 leading to inflammation and hypercoagulability. 18 Several markers of endothelial dysfunction have been reported to be associated with the severity of COVID-19, such as circulating CD31. 18 However, the timepoint during disease progression when endothelial dysfunction becomes more pronounced is not certain. The results of this study not only confirmed that endothelial dysfunction is associated with COVID-19 (by showing that the levels of EV-expressing CD31 and PSGL-1 are increased in patients), but also demonstrated that these markers are not able to discriminate severe COVID-19 from cases at the beginning of the disease.
Part of the COVID-19-associated coagulopathy is attributed to platelet activation, 19 which is also associated with disease severity and increased risk of death. 20 Again, it is not known when platelet activation becomes pathological during the clinical course of COVID-19. The increased levels of EVs expressing CD41A in patients highlights the occurrence of platelet activation; however, these platelet-derived EVs were associated with COVID-19 severity only in the late stages of the disease.
COVID-19-associated coagulopathy has also been attributed to the activation and hyperexpression of TF. 21 Several studies on EVs in COVID-19 have evaluated the levels and procoagulant activity of EVs expressing TF because of its role in blood coagulation. 22 Previous studies showed that EV-TF+ (or EV-CD142+) levels are not only increased in COVID-19 patients, 21 but are also higher in patients with severe COVID-19 compared to moderate cases 6 and are associated with a hypercoagulable state. 23 In this study, we confirmed that severe COVID-19 is associated with increased levels of EV-TF+, which in turn is associated with inflammation and hypercoagulability. However, these associations were observed only in later stages of the disease.
Identifying patients at higher risk of developing severe COVID-19 is important for appropriate treatment and optimal use of resources. However, for this to be possible, prognostic markers are needed at early stages of the disease. Several studies have shown that laboratory parameters, such as lymphocyte and neutrophil counts, lactate dehydrogenase, CRP, D-dimer, and their interactions with demographic and comorbidity factors, are strong predictors of COVID-19 severity and risk of death. 24-28 Our study confirms that D-dimer and CRP levels are early markers of COVID-19 severity, as both were elevated in severe forms of the disease from the first weeks of symptoms. In contrast, EV levels were significantly higher in severe COVID-19 than in mild cases later during disease progression, suggesting that the release of EVs expressing inflammatory markers and TF is a late phenomenon during the progression of COVID-19 to severe forms. From this perspective, the release of EVs from activated platelets and endothelial cells, as well as procoagulant EVs expressing TF are probably a consequence of the exacerbation of the inflammatory response.
It is also important to note that EV levels remain high (above control reference levels) in individuals with mild and severe COVID-19 for up to one month after the onset of COVID-19 symptoms. COVID-19 represents an enormous health burden, not only because of the severe forms of acute infection, but also because of the long-term symptoms after COVID-19 infection (long COVID). 29 Symptoms of infection may persist for months, starting from one to three months after the onset of COVID-19 and lasting at least two months, 29,30 and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, cough, loss of taste, paresthesia, and cognitive dysfunction. 29,31 It has been reported that up to 20% of patients complain of long COVID symptoms of any severity and vaccination decreases the odds of long COVID. 32
In long COVID, it is possible that multiple mechanisms, such as persistence of viral antigens, lead to chronic activation of the immune system. 33 The results of this study showed that EV levels correlated with inflammatory markers both in 3 and 4 weeks after symptom onset. Therefore, these findings suggest that a chronic inflammatory state may contribute to the continued release of EVs into the circulation.
Some limitations of this study should be pointed out. First, pre-analytical conditions are important sources of variability in the results and include blood collection, plasma separation, sample storage and EV separation. Blood collection and plasma separation were performed by different healthcare professionals and not by the researchers, which may have resulted in pre-analytical variability. Second, all demographic and clinical data reported in this manuscript were obtained from medical records; therefore, it cannot be excluded that some information is missing. Third, even though it was not confirmed whether EV-TF+ has procoagulant activity, it was demonstrated that EV-TF+ is a biomarker for the progression and severity of COVID-19. Fourth, the number of patients included is limited, resulting in large confidence intervals and imprecise results. Despite the small sample size, the study demonstrated the differences in EV levels between COVID-19 patients and controls and between disease severities. Finally, the patients were not followed up for more than 30 days, so it is not possible to determine how long EVs remain high in circulation and whether they contribute to the symptoms of long COVID-19.
ConclusionEVs expressing TF, platelet-endothelium and leukocyte-platelet adhesion antigens are involved in the inflammatory process of both mild and severe COVID-19. The release of EVs increases as COVID-19 progresses to more severe forms, combined with increased inflammation and hypercoagulability, and remains high for at least 30 days from the onset of the disease. By suggesting that EV release is a consequence of the exacerbation of the inflammatory response, these findings contribute to the understanding of the pathogenesis of acute, and possibly long, COVID-19.