Blood transfusion is an effective therapeutic practice. However, even adopting all procedures for transfusion safety, there are risks, one of which is immediate adverse reactions. The aim of this study was, by active search, to evaluate the occurrence of immediate adverse reactions estimating the occurrence rate within the first 24 h.
MethodsAn exploratory, descriptive, prospective study with quantitative analysis was carried out of patients undergoing surgery who received blood component transfusions during hospitalization from October 2018 to August 2019. Data on blood component request forms were collected from the transfusion agency by reviewing medical records and interviewing the patient or family members. Descriptive statistics and the chi-square test were used to analyze the association of demographic variables with the presence or absence of transfusion reactions.
ResultsA total of 1042 blood component units were transfused in 393 transfusions performed on 184 patients. The main transfused blood component was packed red blood cells. Seventeen reactions were identified in the medical records, using the active search method, none of which had been reported. The transfusion reaction rate was 16.3 occurrences per 1000 transfused units, while the notification rate for the 9389 blood component units transfused by the transfusion agency in the study period was 3.83/1000. There was no statistically significant association between the occurrences or not of transfusion reactions and demographic variables.
ConclusionThrough the active search method, it was possible to observe the underreporting of adverse reactions, showing inadequate compliance with current legislation, which is essential to minimize errors and increase transfusion safety.
Even with all the advances in surgical and anesthetic techniques, the use of blood components constitutes and irreplaceable therapy and plays a relevant role in maintaining the lives of patients undergoing surgery.1-3
Despite adopting all the operational procedures and the patient safety policies, transfusion reactions (TR) can occur during or after the act of transfusion. In addition, the hospital surgical scenario involves complex care processes, which make the patient more susceptible to adverse events.4 In Brazil, according to the Brazilian Health Regulatory Agency (ANVISA), TR are defined as an undesirable effect or response observed in a person, temporally associated with the administration of blood or blood components; they are classified according to the time of onset of the clinical condition as immediate (IAR) or late adverse reactions and as mild, moderate, severe or death linked with the transfusion.5 The following IAR stand out: non-hemolytic febrile reactions (NHFR), allergic reactions (AR), acute hemolytic reactions (AHR), transfusion-associated circulatory overload (TACO), transfusion-associated dyspnea (TAD), bacterial contamination reactions and transfusion-related acute lung injury (TRALI).6
According to the Brazilian hemovigilance system, the notification of transfusion reactions has been mandatory since 2010,7 and with another ruling (RDC N° 34), the establishment of transfusion committees, with the attribution of monitoring hemotherapy, promoting the rational use of blood and hemovigilance, became compulsory in 2014.8,9 Underreporting of transfusion reactions is common in Brazil and worldwide. In 2014, ANVISA's Hemovigilance Bulletin No. 7 described a rate of 2.83/1000, with large variations between different regions, such as 0.0/1000 in the state of Amapá (north of Brazil), 9.4/1000 in Federal District/Brasília (Midwest) and 1.6/1000 in Minas Gerais, (southeast).9,10 These results show great disparities between the different regions of the country and, certainly, high rates of underreporting.
The Hospital das Clínicas of the Federal University of Triângulo Mineiro (HC-UFTM) is situated in Uberaba, Minas Gerais, Brazil; it serves all the cities of the South Triangle region. In 2017, monitoring carried out by the HC transfusion committee evaluated 18 transfusion processes, of which, 48 % did not comply with the institution´s hemotherapy protocol.11 In the same year, the TR rate was 1.9/1000 and in 2018, it had increased to 2.25/1000 transfused units. The HC-UFTM Transfusion Committee comprises nominees of Uberaba's Regional Blood Bank and HC-UFTM.12 The hemovigilance actions at the HC-UFTM has detected many non-conformities related to routines and established protocols in the transfusion process. It is believed that there is an underreporting of transfusion-related adverse events.11
In respect to this, the important role of the nursing team is stressed, as it actively participates in the entire blood transfusion cycle, especially in the act itself. Knowing the unique aspects of transfusion incidents, the team must be aware of the particularities of transfusional incidents in order to better identify and notify their occurrence, guaranteeing safe and quality care, and reducing the risks and harm to patient's health.10,13 Thus, the present study aimed to evaluate, by active search, the occurrence of IAR and estimate the rate of their occurrence in a teaching hospital.
Material and methodsThis exploratory, descriptive and prospective study using a quantity approach based on field research was approved by the Research Ethics Committee of HC-UFTM (protocol number 2.894.473).
Sample characterizationBetween October 2018 and August 2019, a group of 184 surgical patients who received blood component transfusions during hospitalization in the sectors of surgical clinic, surgery center, orthopedics, adult emergency room, post-anesthesia recovery room, adult intensive care unit (ICU-A) and coronary intensive care unit (ICU-C) were studied.
Inclusion and exclusion criteriaAfter a Free and Informed Consent Form was signed by the patient or family member, the patients were included in this study according to the following criteria: age equal to or greater than 18 years who received blood component transfusions. Patients were excluded when blood components were requested but not transfused, as were those who were hospitalized in other sectors such as on the neurological and gynecological surgery wards (due to the small number of transfusions), those who died within the first 24 h and under 18-year olds.
Data collectionData collection was carried out by active search of patient records, interviews with patients or their family members and the analysis of blood component requests in the transfusion agency 24 h after the procedure. The data collected included demographic (gender, age and skin color self-declared) and clinical-epidemiological (hospitalization sector, surgical specialty, indication for transfusion, type and quantity of blood component, occurrence, type and severity of IAR) variables, as well as the nursing team reports with the records of the care provided. Transfusion reactions were classified according to the Conceptual and Operational Framework for Hemovigilance.5
Subsequently, these data were compiled in a Microsoft Office Excel 2010® spreadsheet designed for this research.
Statistical analysisThe data were processed using a double-entry process in the Statistical Package for the Social Sciences (SPSS version 21.0®) program. Descriptive analysis of absolute and relative frequencies, measures of central tendency (mean) and dispersion (pattern deviation) are reported.
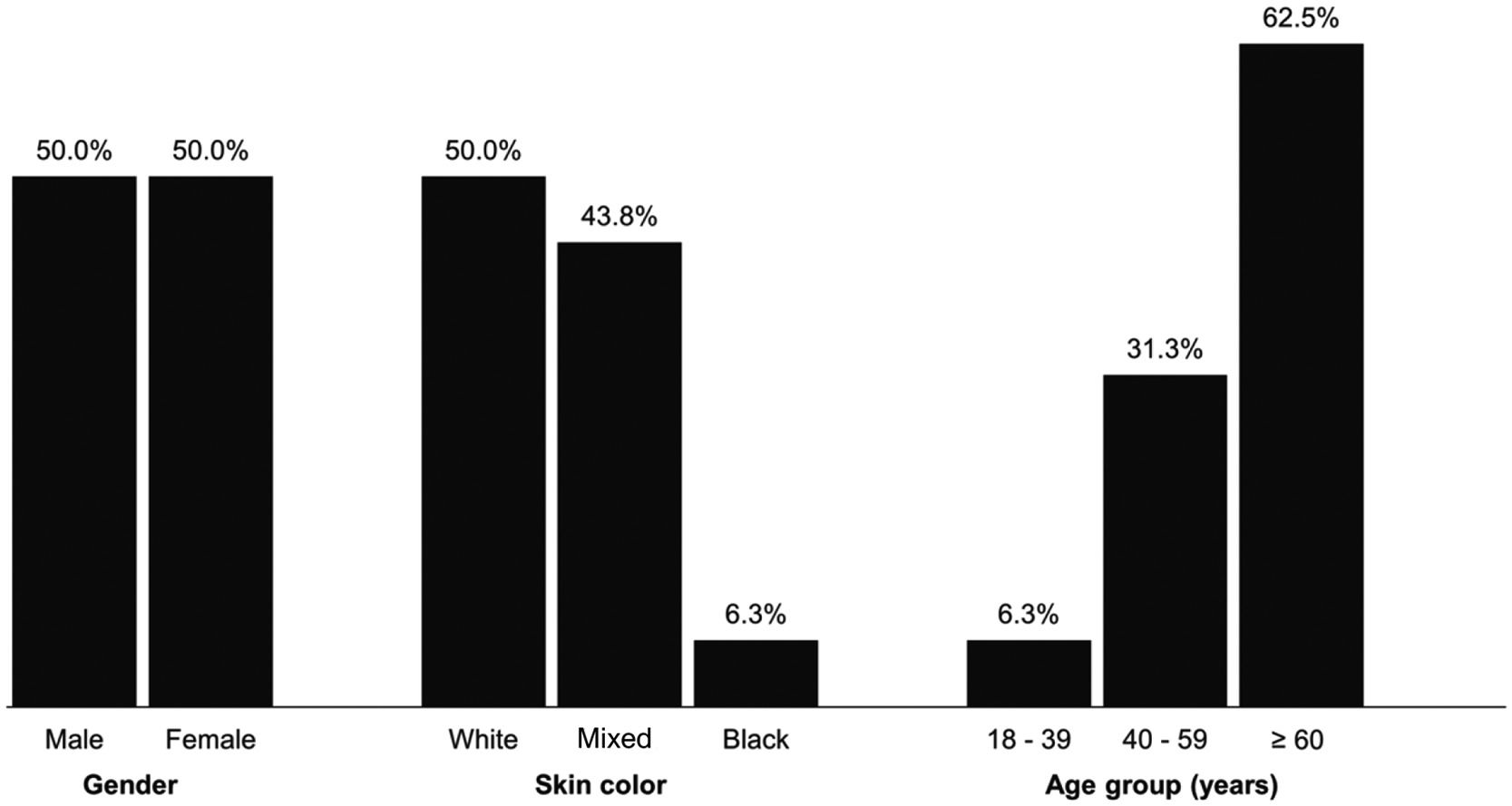
ResultsA total of 393 transfusions took place in 184 patients who received 1042 blood component units representing 11.1 % of the 9389 transfusions performed by the transfusion agency in the period. Of these 184 patients, 53.8 % were male and 46.2 % were female. Regarding skin color, 52.7 % were white, 38 % mixed and 9.2 % black. As for age, 53.8 % of the patients were 60 years old or older, 26.6 % were between 40 and 59 years old and 19.6 % were between 18 and 39 years old (57.74 ± 19.21 - Figure 1).
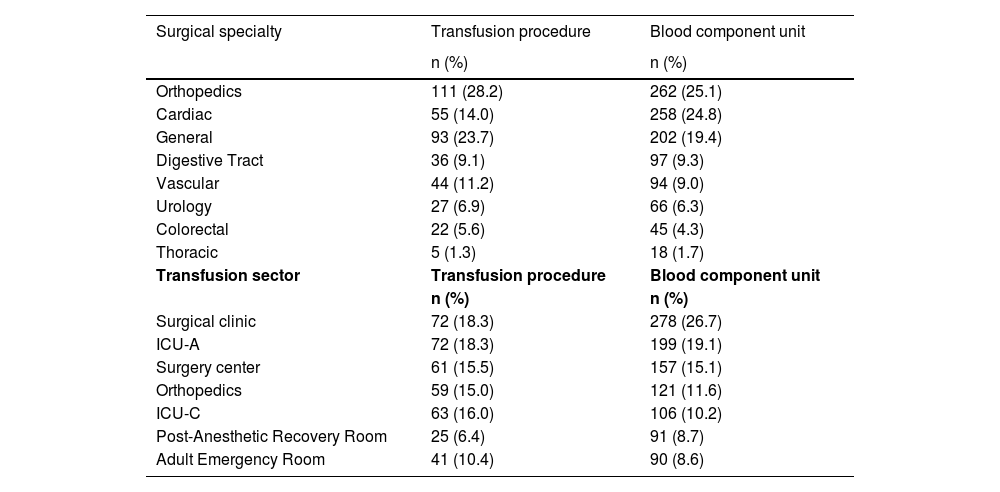
The specialties that required the most transfusions were orthopedics, cardiac surgery and general surgery, representing 25.1 %, 24.8 % and 19.4 %, respectively, of the transfused blood components. The sectors that most transfused blood components were the surgical clinic (26.7 %) and ICU-A (19.1 %), followed by surgery center (15.1 %) and orthopedics (11.6 % - Table 1).
Distribution of 393 transfusion events and 1042 blood components transfused according to surgical specialty and place performed.
ICU-A: adult intensive care unit; ICU-C: coronary intensive care unit.
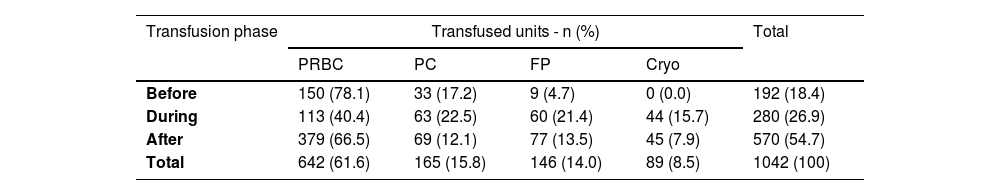
About the timing of transfusions, 18.4 %, 26.9 % and 54.7 % were done before, during and after the surgery, respectively. Packed red blood cells (PRBC) were the most transfused blood component (61.6 %), followed by platelet concentrates (PC - 15.8 %), fresh plasma (FP - 14 %) and cryoprecipitate (Cryo - 8.5 %) (Table 2).
Distribution of the 1042 transfused blood components according to the transfusion phase.
PRBC: packed red blood cells; PC: platelet concentrates; FP: fresh plasma; Cryo: cryoprecipitate.
Seventeen IAR were identified by active search in 16 patients during the 393 transfusions (1042 transfused units); this corresponds to 16.3 reactions for every 1000 transfused blood component units. In this period, of the 9389 blood components transfused, 36 IAR were reported to the Transfusion Agency (3.83/1000). However, none of the 17 reactions identified in the active search had been recorded and reported.
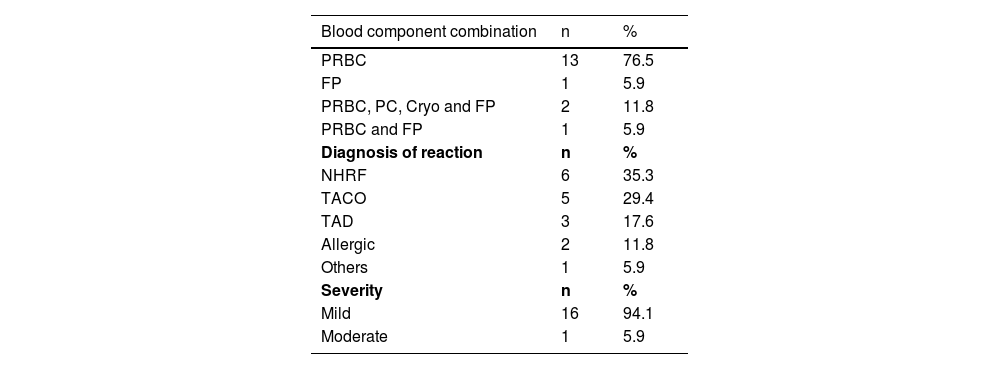
A total of 58.8 % of transfusion reactions occurred between 30 min and 12 h after the end of the procedure, and those during the transfusion process itself occurred between 30 min and seven hours after its beginning. Most (76.5 %) of the reactions occurred in patients who had received only PRBC; in three (17.6 %) this blood component was part of the transfused hemotherapy arsenal. NHFR was the most frequent IAR (35.3 %), followed by TACO in 29.4 % of cases. As for severity, 94.1 % of the reactions were mild with only one (5.9 %) being moderate. (Table 3).
Characterization of transfusion reactions regarding the type of blood component, diagnosis and severity.
PRBC: Packed red blood cell; FP: Fresh plasma; PC: platelets concentrate; Cryo: Cryoprecipitate; NHRF: Non-hemolytic febrile reaction associated dyspnea; TACO: Transfusion-associated circulatory overload; TAD: Transfusion.
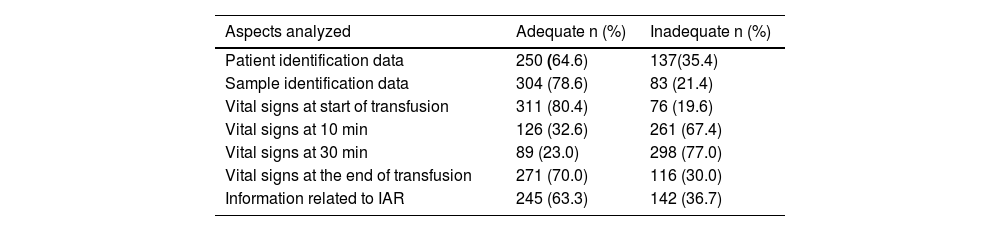
As for the correlation between transfusion and NHFR, TAD and other reactions, it was 100 % classified as probable; regarding TACO as 40 % probable and 60 % possible, and of the two allergic reactions, one was confirmed, and the other was probable. As for the quality of the work carried out by the nursing team in hemotherapy assistance, evaluated through the records of the assistance provided in 387 transfusion events, some inadequacies were observed in 92.8 % of the cases. The main non-conformities were in relation to the monitoring of vital signs at 10 and 30 min after the transfusion, which were absent in 72.2 % and 83 % of the patients’ records, respectively, as well as information related to 36.7 % of the IAR (Table 4).
Evaluation of the adequacy index in completing the blood transfusion checklist by the nursing team.
IAR: Immediate adverse reactions.
Through an active search of the medical records of patients treated at the HC-UFTM from October 2018 to August 2019, this study demonstrates that IAR were underreported. Safe practices related to blood transfusion procedures have become an urgent necessity,14 because they are crucial to guarantee the safety of the patient.2 Among these practices, those related to the care provided by the nursing team in the monitoring and recording of the entire transfusion process, from the receipt of the blood component to 24 h after the transfusion, are highlighted, aiming at the prevention, detection, treatment and notification of transfusion-related adverse events.8
The most frequently identified transfusion reactions in the present study were NHRF, followed by TACO and TAD, as reported by other studies.15-17 Data from the European system for reporting serious adverse events indicate that TACO is the most frequently reported reaction, followed by NHFR and allergic reactions.17 However, the international hemovigilance database found that allergic reactions were the most common, followed by NHFR18 and in a recent study of a Chinese group, allergic reactions represented 73.2 %, followed by TACO and NHFR.19
In a report from an international hemovigilance database, 75 % of transfusion reactions were classified as mild.18 Data from the Brazilian Health Ministry in 2014, show that IAR were reported as mild in 82.6 % of cases, moderate in 14.3 %, severe in 2.8 % and death in 0.3 %.10 In this study, most of the reactions identified were classified as mild (94.1 %) with only one (5.9 %) being moderate, results that corroborate the national and international data.10,18 PRBC were the blood component most frequently linked to IAR. This fact can be explained as it was the most used component, representing almost two thirds of the transfused blood components and it was a part of almost all transfusion procedures in which other blood components were also used. Different rates of IAR have been reported in several other studies. Between 2008 and 2015 in São Paulo, Brazil, the rate varied between 3.44 and 4.55/1000 transfused blood component units.20 A study by the National Healthcare Safety Network Hemovigilance Module in the United States reported 2.2/100021, a study from southern India reported 9.5/100022 and another from the province of Fujian, also in India, found a rate of 14/1000.23
The high occurrence of IAR documented in this active search (16.3/1000) is noteworthy, especially since none of them were recorded in the medical records or reported to the hemotherapy service. As in the study period, 36 acute adverse reactions (3.83/1000) were reported after 9389 transfused blood components, it was expected that at least four IAR should have been registered. Furthermore, the fact that records reported the absence of adverse reactions in 63 % of transfusion procedures, strongly suggests that many of the 17 IAR identified in the active search occurred during these procedures but were not registered. This fact, in addition that no record was made of adverse reactions in more than a third of transfusions, demonstrates the lack of knowledge of their manifestations and/or the lack of commitment in respect to this type of event.
In a study from Namibia, the authors found a rate of 11.1/1000 during an active search for IAR. Of 28 reactions identified, only one had been reported to the surveillance system, reinforcing our findings of high rates of underreporting of IAR.24 None of the studies evaluated presented rates as high as the 16.3/1000 identified here. Only in this case and in the study by Meza et al.,24 the data were obtained through active searches and not reports sent to the respective hemovigilance services and/or obtained from hospital records, demonstrating that underreporting of transfusion reactions is still a global challenge.
Thus, the results of this study not only demonstrate very high levels of underreporting of acute adverse transfusion events in this hospital, but also strongly suggest that the same probably occurs in most other hospital services where transfusion procedures are performed. The only other Brazilian study in which an active search for IAR was carried out, registered as a master's dissertation, found even higher rates (91.8/1000),17 which reinforces the results obtained that underreporting is not isolated.
The low reporting rates can be attributed to the professionals' lack of knowledge regarding the signs and symptoms of a reaction, the inability to link these signs and symptoms to the infusion of blood components at the end of the transfusion as some signs are nonspecific and non-compliance with the rules and regulations governing hemotherapy. These situations reflect flaws in the training programs of the teams and/or the non-existence or inefficiency of transfusion committees and the hemovigilance system, mandatory in the Brazilian legislation as in other countries. The transfusion procedure encompasses several distinct steps, which are often performed by different individuals.25 The high rates of non-compliance registered and the large variations in the rates of IAR observed between different countries and, in particular, in different hemotherapy services in the same country, demonstrate that the gaps in transfusion processes are still a reality not only in developing countries and that they prejudice transfusion safety.
In this study, some limitations were encountered, such as: the forms used in the institution were not always available and adequately filled out, the low adherence of professionals to obtaining consent in relation to transfusions and that the indications for blood components were not always in accordance with the rational use protocol of blood. The HC-UFTM Transfusion Committee has been facing challenges, like other Brazilian hospitals, in the proper performance of functions such as coordination, guidance and surveillance of hemotherapy activities.
ConclusionThe active search in this study demonstrated the existence of a high level of underreporting of IAR in the evaluated service, showing inadequate compliance with current legislation, which is critical to minimize the occurrence of errors. Hemotherapy committees and well-structured and active hemovigilance programs are essential to minimize errors and ensure transfusion safety.
The large variations in IAR rates between different countries and, in particular, in different hemotherapy services in the same country, showed that underreporting of these events is still a reality, not only in developing countries. This situation highlights the urgent need for hemotherapy services and medical and nursing teams in hospitals to comply with the legislation that regulates hemotherapy activities. The effective performance of the hemotherapy committees and in-hospital hemovigilance programs is also fundamental. Such measures are crucial to minimize risks and increase transfusion safety.
Funding statementThere is no funding statement.
Ethics approval statementThis study was approved by Research Ethics Committee of Hospital das Clínicas from Federal University of Triângulo Mineiro (HC-UFTM), protocol number 2.894.473.
Patient consent statementThe patients were included in study after the signing of a Free and Informed Consent Form by patient or family member.
Permission to reproduce material from other sourcesNot applicable.
Clinical trial registrationNot applicable.