Blood transfusion is a life-saving procedure, but may cause adverse transfusion reactions (TRs). The reporting of TRs is often missed due to various reasons. The aim of this study was to determine the incidence of unreported acute TRs through active surveillance and to compare it with the incidence of passively reported TRs.
MethodsThis prospective observational study was done over a period of four months at a tertiary care hospital. A total of 500 consecutive transfusion episodes (TEs) in patients who had received blood component transfusions in the intensive care units were included in the study. Comprehensive data were collected from the blood bank records, patient records and through interviews with the attending clinical staff. The TEs were defined as all blood components issued to a single patient in 24h.
ResultsThe overall incidence of TRs was 1.8 % (9 cases), with 0.4 % (2 cases) being reported passively, while 1.4 % (7 cases) were identified during active surveillance. The transfusion-associated cardiac overload (TACO) had the highest incidence of 1.2 % (6 cases) in active surveillance. A single case of acute hemolytic transfusion reaction was also observed during active surveillance. The passively reported TRs were one allergic reaction and one febrile non-hemolytic transfusion reaction.
ConclusionActive surveillance of TRs provided an insight into the true incidence of TRs, which is higher when compared with the passively reported TRs. The TACO was found to have the highest incidence and not a single case was reported. There is a need to improve awareness regarding TR reporting.
The transfusion reaction (TR) can be defined as an unintended response in a patient to the transfusion of blood components which prolongs hospitalization, is disabling or incapacitating and increases morbidity or causes mortality.1 The TRs can be broadly classified as (i) acute or delayed (depending upon the time of occurrence) and (ii) immune or non-immune (depending upon the pathophysiology).2–4
It has been estimated that up to 10 % of all blood transfusions are associated with an adverse event.5 These events must be reported to the blood transfusion services (BTS) by the clinicians. One of the main goals of developing a hemovigilance program (HvP) is to improve the reporting of transfusion reactions and data collection, followed by evidence-based improvements in blood transfusion practices. Hemovigilance was first launched in France in 1994 and followed by other countries.6 The hemovigilance program of India (HvPI) was launched in 2012.7 The HvPs rely on passive reporting of TRs by clinicians to the transfusion services that upload the data on the national portals.7–11 Active surveillance uniformly reports higher rates of TRs than the routine passive reporting systems.12–15
The incidence of actual TRs remains underestimated and the various reasons for this are: (i) lack of awareness regarding TRs among clinicians, (ii) delayed reactions being missed as the temporal relationship with transfusion is overlooked, (iii) reporting of TRs, such as the transfusion-associated circulatory overload (TACO) and transfusion-related acute lung injury (TRALI), does not change the course of management, (iv) difficulty in identifying TRs in unconscious patients and (v) overlapping signs and symptoms in the patient’s underlying condition may lead to missed reactions.
Allergic reactions, the febrile non-hemolytic transfusion reaction (FNHTR) and hemolytic transfusion reactions (HTR) are the usually reported TRs, while others remain underreported.15,16
In accordance with previously published studies which state that TRs are often missed,12–14 we hypothesized that the current reporting system at our hospital failed to capture all the TRs. Therefore, we did active surveillance to determine the incidence of missed TRs and compare the incidence and category of TRs identified by active surveillance to the records of passively reported cases.
Materials and methodsThis prospective observational study was conducted in the Department of Transfusion Medicine at a tertiary care liver institute in New Delhi over a period of 4 months from June 2018 to September 2018. Another two months were required for data analysis. Patients admitted to the liver coma intensive care unit (LC-ICU), transplant ICU and high dependency unit (HDU) were included in the study, while patients undergoing plasma exchange and those under 18 years old were excluded. A total of 500 consecutive transfusion episodes were followed up on for 24h post-transfusion during the study period. For study purposes, a transfusion episode was defined as all the blood components being transfused to a patient in 24h.
We made a comprehensive review of the patient clinical records and interacted with the patient clinical team to detect cases under suspicion for TRs. The data were collected from the blood bank, patient records and clinical staff interviews. Data included patient demographics (age, gender, diagnosis, etc.), component-related data (type and number of components,), pre- and post-transfusion vitals (blood pressure, heart rate, respiratory rate, oxygen saturation) laboratory parameters (blood grouping, direct Coomb's test, indirect Coomb's test, pre- and post-transfusion complete blood count, pre- and post-transfusion renal function test, pre- and post-transfusion liver function test) and radiology reports (pre- and post-transfusion chest X-ray) and clinical records/interaction.
In the case that a TR was detected, the following data were collected: reporting status of the TRs (whether reported to transfusion services or not), the causative component responsible for the reaction, category of the reaction, the severity of the reaction, the imputability and outcome (whether resolved, resolved with consequences or death occurred). All of the TRs were adjudicated, as per defined by the International Society of Blood Transfusion (ISBT) working party on hemovigilance.2 The severity was graded and the imputability was assessed, as per defined below.
Severity:
Grade 1 (Non-Severe): the recipient may have required medical intervention (e.g., symptomatic treatment), but lack of this would not have resulted in permanent damage or impairment of a body function.
Grade 2 (Severe): the recipient required in-patient hospitalization or prolongation of hospitalization directly attributable to the event and/or the adverse event resulted in persistent or significant disability or incapacity, or the adverse event necessitated medical or surgical intervention to preclude permanent damage or impairment of a body function.
Grade 3 (Life-threatening): the recipient required major intervention following the transfusion (vasopressors, intubation, transfer to the intensive care) to prevent death.
Grade 4 (Death): the recipient died following an adverse transfusion reaction.
Imputability:
This is, once the investigation of the adverse transfusion event (ATE) is completed, the assessment of the strength of the relation between the transfusion and the ATE.
Definite (certain): when there is conclusive evidence beyond reasonable doubt that the adverse event can be attributed to the transfusion.
Probable (likely): when the evidence is clearly in favor of attributing the adverse event to the transfusion.
Possible: when the evidence is indeterminate for attributing the adverse event to the transfusion or an alternate cause.
Unlikely (doubtful): when the evidence is clearly in favor of attributing the adverse event to causes other than the transfusion.
Excluded: when there is conclusive evidence beyond reasonable doubt that the adverse event can be attributed to causes other than the transfusion.
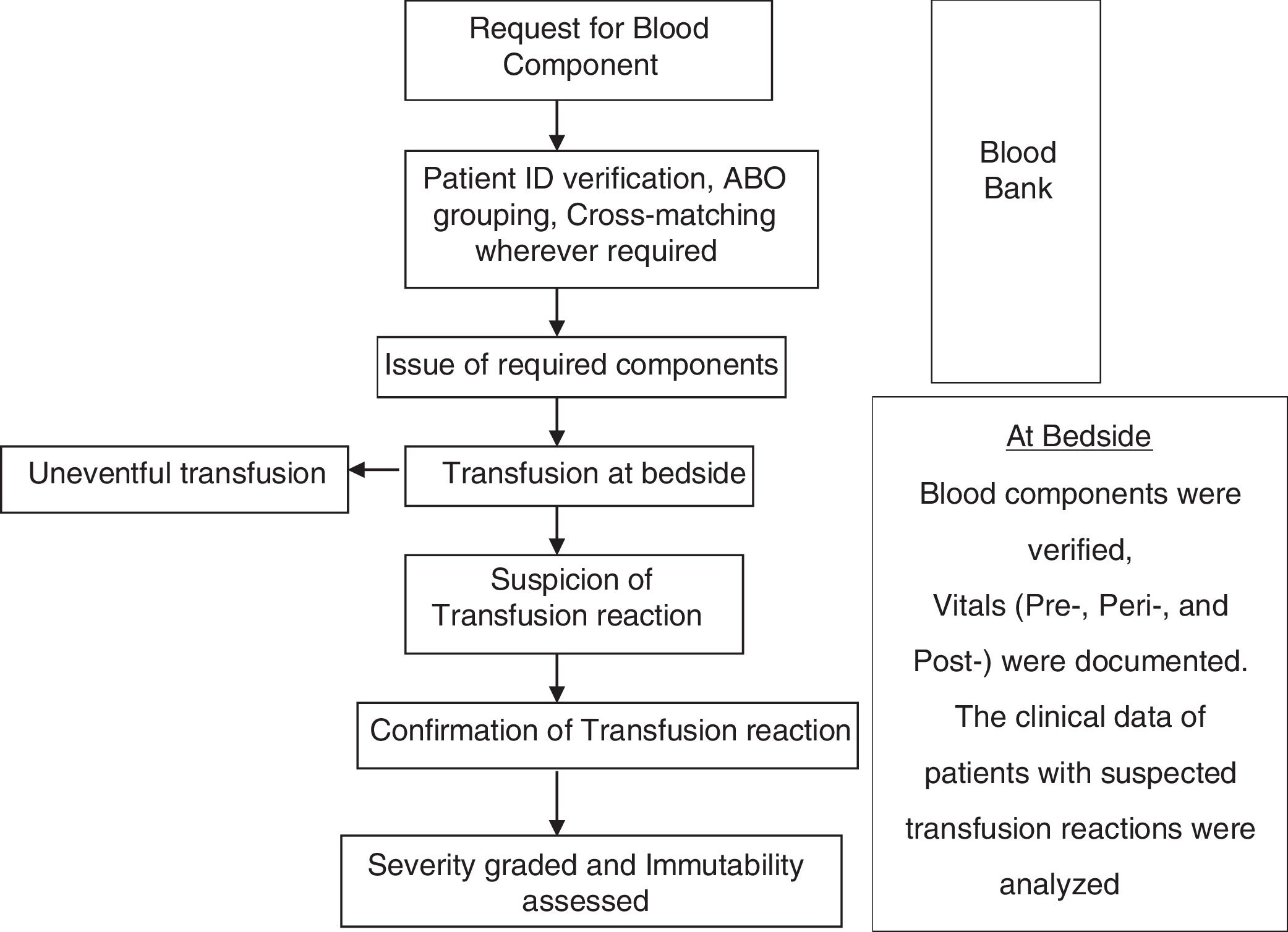
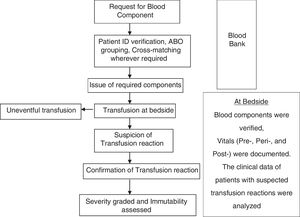
At the end of the study period, the prevalence of TRs identified during active surveillance was compared with the prevalence of passively reported TRs (Fig. 1). summarizes the study algorithm.
StatisticsAll the collected data were entered into a Microsoft Excel spreadsheet (MS Office). Descriptive statistics were expressed as mean with standard deviation and median and range were used for continuous variables, while the absolute number and percentage were used for categorical variables. Incidence was calculated as the total number of transfusion reactions divided by the total number of transfusion episodes.
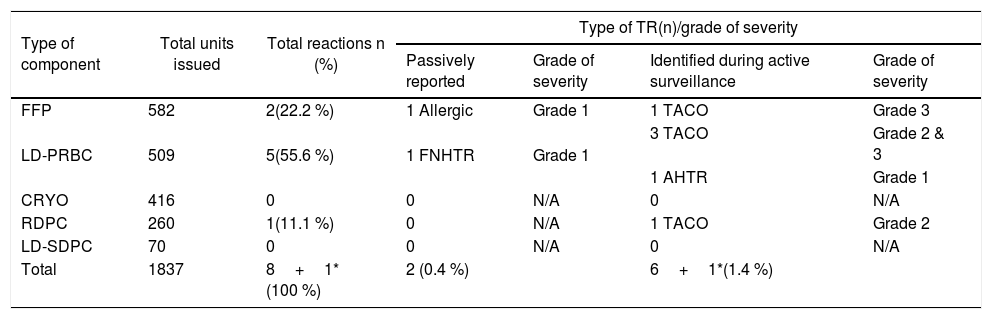
ResultsDuring the study period, a total of 500 consecutive transfusion episodes were analyzed in 177 patients with a median age of 46 years (149 males, 84 %; 28 females, 16 %). The average number of transfusion episodes was 2.82 and an average of 10.38 blood component units were transfused per patient. Seventy-four (41.8 %) patients had a single transfusion episode. A total of 1,837 blood components were transfused, of which 31.7 % (582 units) were fresh frozen plasma (FFP) followed by leucodepleted packed red blood cells (LD-PRBCs), which accounted for 27.7 % (509 units) of transfusions (Table1).
Types and severity of acute transfusion reactions seen with different blood components.
| Type of component | Total units issued | Total reactions n (%) | Type of TR(n)/grade of severity | |||
|---|---|---|---|---|---|---|
| Passively reported | Grade of severity | Identified during active surveillance | Grade of severity | |||
| FFP | 582 | 2(22.2 %) | 1 Allergic | Grade 1 | 1 TACO | Grade 3 |
| LD-PRBC | 509 | 5(55.6 %) | 1 FNHTR | Grade 1 | 3 TACO | Grade 2 & 3 |
| 1 AHTR | Grade 1 | |||||
| CRYO | 416 | 0 | 0 | N/A | 0 | N/A |
| RDPC | 260 | 1(11.1 %) | 0 | N/A | 1 TACO | Grade 2 |
| LD-SDPC | 70 | 0 | 0 | N/A | 0 | N/A |
| Total | 1837 | 8+1*(100 %) | 2 (0.4 %) | 6+1*(1.4 %) | ||
N/A: Not Applicable.
N.B. 1*: One TACO case was associated with transfusion of multiple components.
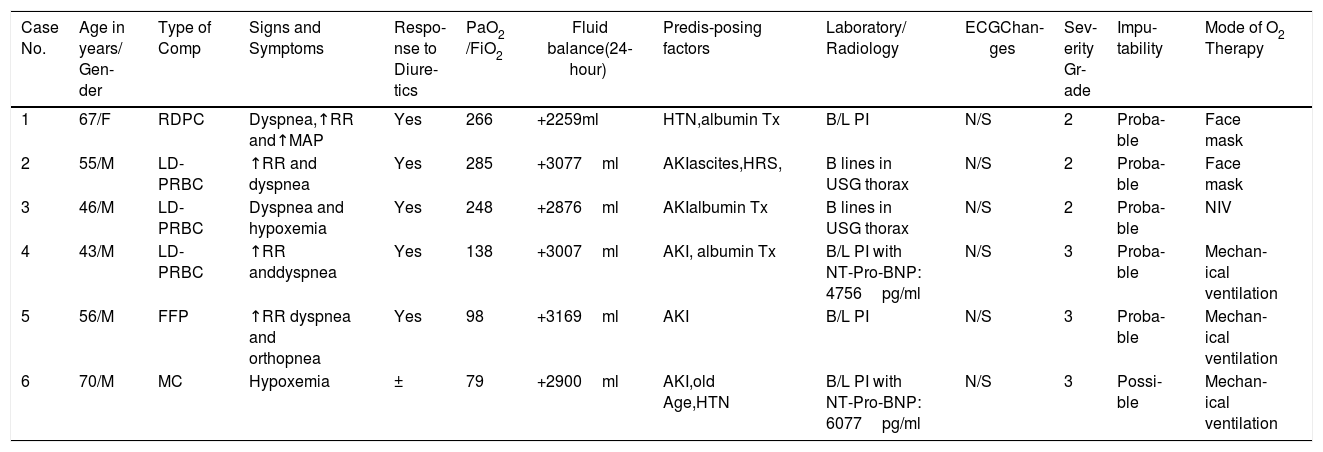
A total of 9 transfusion reactions occurred during this period. The LD-PRBCs were responsible for the majority (5 cases; 55.6 %) of transfusion reactions identified (Table 1). Of these, only 2 (22.2 %) were reported, while 7 (77.8 %) remained unreported and thus, the overall incidence of transfusion reactions was 1.8 % per transfusion episode (1.4 % unreported; 0.4 % reported) and 0.5 % per component transfused (0.4 % unreported; 0.1 % reported).The transfusion reactions and the implicated components are shown in Table 1. The passively reported transfusion reactions were one FNHTR and one allergic transfusion reaction. These TRs had a clear temporal association with the component transfused. The most commonly encountered transfusion reaction during active surveillance was the TACO, which was seen in 6 patients. Five TACO cases showed a clear temporal association with one type of component and in one case, the patient had received multiple types of components (Table 1). All the cases of TACO showed symptoms of hypoxemia and radiological features suggestive of fluid overload that responded to the administration of diuretics except in one case in which the patient was already on diuretics and the imputability was considered ‘possible’ for this case (Table 2). One case of an acute hemolytic transfusion reaction (AHTR) was also observed during active surveillance in which the patient was transfused with one unit of LD-PRBC, following which the hemoglobin level fell below the pre-transfusion level and the concerned clinician sent an independent request for a direct antiglobulin test (DAT) and an indirect antiglobulin test (IAT), without reporting the TR. The DAT and IAT were positive, but the antibody causing hemolysis could not be identified. It was an extravascular hemolysis and occurred within 24h of the LD-PRBC transfusion due to an anamnestic response to a pre-existing alloantibody.
Clinical Details of TACO Cases.
| Case No. | Age in years/ Gen-der | Type of Comp | Signs and Symptoms | Respo-nse to Diure-tics | PaO2 /FiO2 | Fluid balance(24- hour) | Predis-posing factors | Laboratory/ Radiology | ECGChan-ges | Sev-erity Gr-ade | Impu-tability | Mode of O2 Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67/F | RDPC | Dyspnea,↑RR and↑MAP | Yes | 266 | +2259ml | HTN,albumin Tx | B/L PI | N/S | 2 | Proba-ble | Face mask |
| 2 | 55/M | LD-PRBC | ↑RR and dyspnea | Yes | 285 | +3077ml | AKIascites,HRS, | B lines in USG thorax | N/S | 2 | Proba-ble | Face mask |
| 3 | 46/M | LD-PRBC | Dyspnea and hypoxemia | Yes | 248 | +2876ml | AKIalbumin Tx | B lines in USG thorax | N/S | 2 | Proba-ble | NIV |
| 4 | 43/M | LD-PRBC | ↑RR anddyspnea | Yes | 138 | +3007ml | AKI, albumin Tx | B/L PI with NT-Pro-BNP: 4756pg/ml | N/S | 3 | Proba-ble | Mechan-ical ventilation |
| 5 | 56/M | FFP | ↑RR dyspnea and orthopnea | Yes | 98 | +3169ml | AKI | B/L PI | N/S | 3 | Proba-ble | Mechan-ical ventilation |
| 6 | 70/M | MC | Hypoxemia | ± | 79 | +2900ml | AKI,old Age,HTN | B/L PI with NT-Pro-BNP: 6077pg/ml | N/S | 3 | Possi-ble | Mechan-ical ventilation |
AKI: Acute kidney injury, B/L: Bilateral, Comp: Components, ECG: Electrocardiography, HRS: Hepatorenal syndrome, HTN: Hypertension, MC: Multiple Components, NT-Pro-BNP: N-terminal pro-brain natriuretic peptide, N/S: Not significant, NIV: Non-invasive ventilation, PI: Pulmonary Infiltrate, PaO2/FiO2: Partial pressure of oxygen in arterial blood divided by fraction of oxygen in inspiratory air, RR: Respiratory rate.
The TR identification and management are important aspects of blood safety. The spectrum of TRs ranges from a mild allergy to life-threatening reactions, such as anaphylactic shock, which is possibly fatal. The recognition, reporting, and monitoring of TRs are the key objectives of HvPs. The aim of this is to improve transfusion practice through data-driven evidence-based improvements, with the aim of improving transfusion safety.17 The major hurdles in adequate reporting of TRs include: 1) lack of understanding/ awareness of the HvP among clinicians, 2) lack of a culture of reporting adverse events, 3) fear of punishment and 4) lack of experts/expertise on hemovigilance.18
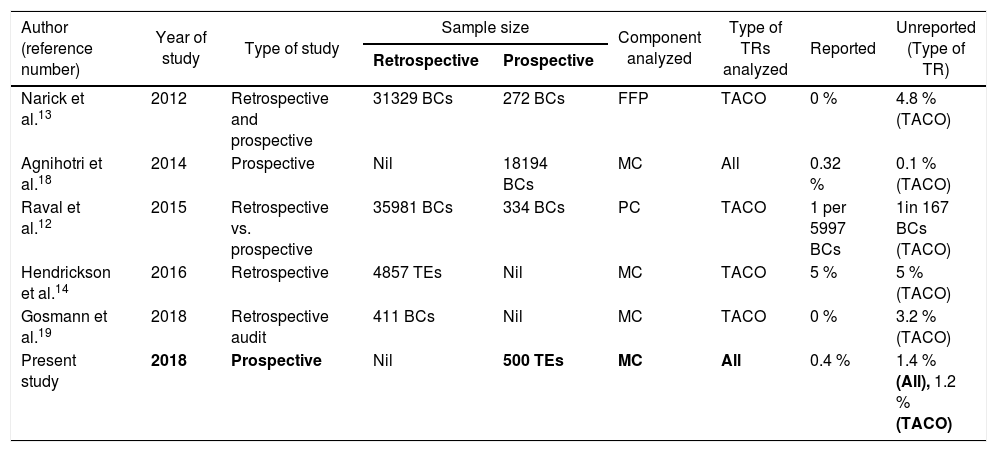
The HvPs rely on passive reporting.7–11 Studies have shown that active reporting systems report higher rates of transfusion reactions than passive ones.13–15 Most studies that have performed active surveillance have done so either by the retrospective review of patient records, the retrospective auditing of electronic hospital medical records, or by active feedback forms.15,19,20 In this prospective study, we performed active surveillance with the detailed review of the patient pre- and post-transfusion vital signs, laboratory variables, radiologic studies and clinical notes, as well as interviews with the patient clinical team within 24h of the transfusion episode, similar to a study done by Narick C et al.14 Published literature13,14 on active surveillance had reported transfusion reactions due to FFP and platelet transfusions, while some studies15,16,19 had included all the blood components transfused, and a similar approach was followed in our study by including all the blood components. Most of the studies13,14,19 that performed active surveillance focused on determining the unknown incidence of the TACO as the unreported TR, but in this study, we performed active surveillance to determine the incidence of all types of unreported acute TRs.
In the present study, we prospectively followed 500 transfusion episodes in adult patients over a 4-month period and found that the incidence of TRs was 1.8 % per transfusion episode, with 0.4 % being reported passively and 1.4 % identified during active surveillance. The true incidence of transfusion reactions was much higher, as compared to passive reporting. Table 3 summarizes a comparison of findings of this study with those of similar studies.
Incidence of acute transfusion reactions on active surveillance- A comparison with published reports.
| Author (reference number) | Year of study | Type of study | Sample size | Component analyzed | Type of TRs analyzed | Reported | Unreported (Type of TR) | |
|---|---|---|---|---|---|---|---|---|
| Retrospective | Prospective | |||||||
| Narick et al.13 | 2012 | Retrospective and prospective | 31329 BCs | 272 BCs | FFP | TACO | 0 % | 4.8 % (TACO) |
| Agnihotri et al.18 | 2014 | Prospective | Nil | 18194 BCs | MC | All | 0.32 % | 0.1 % (TACO) |
| Raval et al.12 | 2015 | Retrospective vs. prospective | 35981 BCs | 334 BCs | PC | TACO | 1 per 5997 BCs | 1in 167 BCs (TACO) |
| Hendrickson et al.14 | 2016 | Retrospective | 4857 TEs | Nil | MC | TACO | 5 % | 5 % (TACO) |
| Gosmann et al.19 | 2018 | Retrospective audit | 411 BCs | Nil | MC | TACO | 0 % | 3.2 % (TACO) |
| Present study | 2018 | Prospective | Nil | 500 TEs | MC | All | 0.4 % | 1.4 % (All), 1.2 % (TACO) |
In passive surveillance, mild non-severe reactions reported, such as febrile and allergic reactions, were 0.2 % each of all the analyzed transfusion episodes; these rates are lower than previously reported studies.21 This incidence is similar to that of the previous 4 years of data reported to the HvPI by the department. The decreased incidence of the FNHTR could be due to the transfusion of universally leucodepleted components. Another possible explanation could be the lean inventory, i.e., the time period between the collection and the issue of components is very short.
During the study period, we did not observe any anaphylactic or hypotensive transfusion reaction.
As per the latest HvPI data, the FNHTRs constituted the most frequently reported TRs (40.84 %),17 but in this study, the TACO was the most common TR observed, constituting 66.67 % of the total transfusion reactions. This incidence was quite high, as compared to that reported in the 2017 SHOT report (44.1 %)22 and in the ISTARE data (4 %).23 The higher proportion of the TACO, compared to other TRs in our study, could possibly be due to the patient cohort having liver diseases that has higher blood component requirements for its underlying coagulopathy and bleeding. These patients are predisposed to volume overload because of the alteration in the renin-angiotensin-aldosterone axis, albumin transfusion and underlining acute kidney injury. The incidence rates of the TACO after transfusion in our study was 1.2 % per transfusion episodes, which is similar to the incidence reported in other active surveillance studies.15,19,20 None of the TACO cases in this study had been reported to the Indian hemovigilance database/HvPI, which reflects the significant gap in the underreporting of transfusion-related adverse reactions, especially the TACO. The present study highlighted that the concerned clinical staff acted promptly upon noticing the clinical signs due to the TACO and provided the required medical care for all the cases; however, they were either not able to recognize its association with a transfusion or did not report it due to the complete absence of impact of reporting on the management of the TACO.
Various factors that lead to underreporting of the TRs should be carefully analyzed and deficiencies should be rectified. Awareness regarding the manifestation of the TRs, as well as the correct identification and proper reporting to the affiliated authority, are imperative for effective hemovigilance. The limitations of this study were the short study period and small sample size, as the study population was confined to three intensive care wards due to logistic issues.
ConclusionsThe active surveillance of transfusion reactions has given us an insight into the true incidence of transfusion reactions. Underreporting of transfusion reactions implies in gaps in communication between treating clinicians and the BTS. The gap can be minimized by the regular continuing education regarding the recognition of suspected TRs and the emphasis on the importance of reporting reactions. Increased awareness regarding the TRs and advantages of reporting them will improve the accuracy of routine reporting and result in an effective hemovigilance, leading to improved patient safety.
Authorship contributionsAS contributed to the concept and design of the study, the data acquisition, analysis and wrote an initial draft of the manuscript. MB contributed to the concept and design of the study, supervised data acquisition and analysis and reviewed and revised the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
The Authors would like to thank Mr. Pankaj Jain, Technical Executive, Department of Transfusion Medicine, Institute of Liver and Biliary Sciences (ILBS) for helping in data collection and preparation of tables and figure.