T-cell acute lymphoblastic leukemia (T-ALL) in children represents a high-risk disease. There is a lack of studies assessing the outcome of T-ALL in Hispanic populations, in which it is a rare malignancy. We report the characteristics and results of treatment for childhood T-cell ALL in children over 14 years at a Latin American reference center.
Material and methodsFrom January 2005 to December 2018, there occurred the analysis of twenty patients ≤ 16 years of age from a low-income open population diagnosed at a university hospital in Northeast Mexico. Clinical and laboratory characteristics, treatment regimens and outcomes were assessed by scrutinizing clinical records and electronic databases. Diagnosis was confirmed by flow cytometry, including positivity for CD-2, 5, 7 and surface/cytoplasmic CD3. Survival rates were assessed by the Kaplan-Meier method.
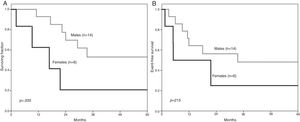
ResultsThere was a male preponderance (70 %), with a 2.3 male-to-female ratio (p = .074), the median age being 9.5 years. Leucocytes at diagnosis were ≥ 50 × 109/L in 13 (65 %) children, with CNS infiltration in 6 (30 %) and organomegaly in 10 (50 %). The five-year overall survival (OS) was 44.3 % (95 % CI 41.96–46.62), significantly lower in girls, at 20.8 % (95 % CI 17.32–24.51) vs. 53.1 % (95 % CI 50.30–55.82), (p = .035) in boys; there was no sex difference in the event-free survival (EFS) (p = .215). The survival was significantly higher after 2010 (p = .034).
ConclusionThe T-cell ALL was more frequent in boys, had a higher mortality in girls and the survival has increased over the last decade with improved chemotherapy and supportive care.
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy that represents approximately 15 % and 25 % of the total childhood and adult acute lymphoblastic leukemia (ALL) cases, respectively.1,2 T-ALL is a neoplasm derived from early T-cell progenitors and is an entity distinctly different from adult T-cell leukemia/lymphoma, which is a mature T-cell neoplasm attributed to the human retrovirus known as human T-cell leukemia virus type I (HTLV-I).3
T-ALL tends to occur in older adolescents with a notably male predominance. It has been associated with a poorer prognosis, compared to B-cell ALL (B-ALL), showing inferior event-free survival (EFS) and overall survival (OS).4 These inferior outcomes are partially explained by the association of the disease with high-risk clinical features, including older patient age, hyperleukocytosis with extramedullary involvement and central nervous system (CNS) infiltration.5,6
Although intensified chemotherapy protocols in high-income countries have increased the OS to 80 % in children with T-ALL, these rates still lag behind its B-cell counterpart.4,6 A relapse in ALL is an event that leads to a dismal prognosis and thus, the T-cell immunophenotype is considered a predictor of inferior post-relapse survival in ALL.7
We were unable to locate reports specifically dealing with the childhood T-ALL incidence, clinical presentation, response to therapy, clinical course and survival rates in the Latin American Hispanic populations. Reports on this group are needed to enhance our understanding of the disease and improve treatment protocols. The objective of the present report was to analyze these variables in children belonging to an open population in Northeast Mexico treated at a referral center over fourteen years.
MethodsThis observational, longitudinal and retrospective study included 20 newly diagnosed T-ALL patients, aged ≤ 16 years attending a single referral center in Northeast Mexico. Eligible patients were those who had complete information in their clinical files and the electronic databases and who had been diagnosed and treated between January 2005 and December 2018 at the Hematology Department of an academic referral center that provides health care for the low-income uninsured open population. Pertinent clinical and biological variables were documented. The study protocol was approved by the Research and Ethics Committee and informed consent was waived.
Diagnosis and relapse criteriaIn addition to a complete medical history and physical examination, the diagnosis of T-ALL, as the first primary malignancy, required a combination of morphology, immunophenotyping and cytogenetic analysis.2 Immunophenotyping was performed by flow cytometry, including positivity for CD-2, 5, 7 and surface/cytoplasmic CD3. Owing to financial restrictions, cytogenetic studies were performed in a minority of patients and thus, were not included.
Lumbar puncture was performed at diagnosis, with CNS infiltration defined as > 5 leukocytes/mm3 with blast morphology in the cerebrospinal fluid. Relapse was defined as the reemergence of the disease and classified as bone marrow, combined or extramedullary. Overall survival was defined as the time from date of diagnosis to death from any cause. Event-free survival was defined as the time from date of diagnosis to the first event of relapse or death. Fluorescence in situ hybridization and polymerase chain reaction (PCR) were used to assess Philadelphia (Ph) chromosome status; flow cytometry minimal residual disease (MRD) studies were performed at the end of induction on day 29 and MRD was considered present if > 0.01 %. Response to steroids was determined using the absolute count of blasts in peripheral blood on day 8 after 7 days of steroid administration. Complete morphological remission was defined as the presence of < 5 % lymphoblasts in bone marrow (BM) on day 36. Organomegaly and lymphadenopathy were documented by clinical examination at the time of diagnosis.
TreatmentThe children were stratified into standard and high-risk groups according to the National Cancer Institute/Rome risk criteria to receive risk-adapted chemotherapy.8,9 Briefly, for children ≤ 16 years during the 2005–2009 period, a protocol based on the LSA2L2 was administered. Induction included standard doses of cyclophosphamide (Cy) prednisone (PDN), vincristine (VCR), adriamycin (ADR) and intrathecal chemotherapy. Consolidation included methotrexate (MTX), cytosine arabinoside (Ara-C) and purinethol (6 M P).10 From 2010 onwards, a BFM-based-scheme was administered. Induction consisted of l-asparaginase, PDN, VCR and ADR. Consolidation included MTX, Ara-C and 6 M P.11
Before 2010, support therapy was limited due to financial restrictions at our hospital, including restricted antibiotic and antifungal choices, as well as the unavailability of apheresis platelets.
Statistical analysisStatistical analyses were performed using the IBM SPSS Statistical software for Windows, Version 22.0 (IBM Corp., Armonk, NY). Since data were nonparametric, descriptive statistics results for continuous variables are presented as medians and ranges. Categorical variables are displayed as absolute numbers and percentages. Demographic and clinical characteristics were compared, using the χ2 test for categorical variables and the Mann–Whitney U test for quantitative variables. Overall survival and EFS were estimated by the Kaplan-Meier method and groups were compared with the log-rank test. The Cox proportional hazard regression model with 95 % confidence intervals (CI) was used for uni- and multivariate analysis. The significance level was set at p < 0.05 for all analyses.
ResultsPatient populationThe study group included 20 patients, representing 5.7 % of 349 ALL cases diagnosed during the study period; there was a male preponderance with 14 males (70 %) vs. 6 females (30 %) (p = .074), with a male-to-female ratio of 2.3. For the whole group, the median age at diagnosis was 9.5 years (2–16); median age for females was 6.5 years (2–14) vs. 10.5 years (2–16) for males (p = 0.628). The patient characteristics are shown in Table 1. The median follow-up was 27 months (2–122).
Clinical and laboratory characteristics of twenty children with T-cell acute lymphoblastic leukemia (T-ALL), aged ≤ 16 years, treated at a referral center in Mexico.
| Characteristics | N (%) |
|---|---|
| Age, median (years), range | 9.5 (2–16) |
| Sex | |
| Male | 14 (70) |
| Female | 6 (30) |
| WBC × 109/L | |
| ≥ 50a | 13 (65) |
| < 50 | 7 (35) |
| Initial CNS involvement | |
| Present | 6 (30) |
| Absent | 14 (70) |
| Mediastinal mass | |
| Yes | 4 (20) |
| No | 16 (80) |
| Adenopathy | |
| Yes | 8 (40) |
| No | 12 (60) |
| Organomegaly | |
| Yes | 10 (50) |
| No | 10 (50) |
| MRD | |
| Positive | 4 (20) |
| Negative | 16 (80) |
Abbreviations: CNS, central nervous system; MRD, minimal residual disease; WBC,white blood cells.
For the whole group, the OS at 5 years was 44.3 % (95 % CI 41.96–46.62), whereas the EFS was 41.5 % (95 % CI 39.22–43.76). The 5-year OS significantly varied by sex; for males, it reached 53.1 % (95 % CI 50.30–55.82) vs. 20.8 % for females (95 % CI 17.32–24.51) (p = .035) (Figure 1). For males, the 5-year EFS was 48.2 % (95 % CI 45.47–55.88) and for females, 25 % (95 % CI 21.10–29.08) (p = .215). The five-year OS and EFS did not significantly differ between age groups; for those aged 1–9 years, the OS was 33.3 % (95 % CI 30.24–36.39) vs. 58.3 % (95 % CI 55.07–61.38) (p = .609) in patients > 9 years, whereas the EFS rates were 35 % (CI 31.88–38.14) and 48 % (CI 44.75–51.17) (p = .572), respectively.
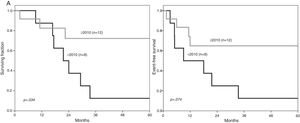
The cohort was divided into two groups based on the date of diagnosis and treatment protocol administered, before and after 2010. Characteristics of these groups are presented in Table 2. Over time, the five-year OS improved significantly (Figure 2A). For children diagnosed before 2010, it was 12.5 % (CI 10.32–14.90), compared to 72.2 % (CI 69.39–74.80) (p = .034) for patients treated from 2010 onwards; in the same period, the five-year EFS was 12.5 % (CI 10.32–14.90) vs. 64.8 (CI 61.92–67.52) (p = .074) (Figure 2B). There were no significant differences in the 5-year OS and EFS when patients with initial organomegaly, adenopathy, CNS infiltration, and positive MRD were compared with those lacking these characteristics (data not shown).
Characteristics and statistical comparison (x2) of 20 patients with T-cell acute lymphoblastic leukemia (T-ALL) in Northeast Mexico between 2005 and 2018.
| Characteristic | < 2010, n (%) | ≥ 2010 n, (%) | pa |
|---|---|---|---|
| Total | 8 (40) | 12 (60) | .371 |
| Median age, years (range) | 5 (2–14) | 11 (2–16) | .650 |
| Sex | |||
| Male | 5 (62.5) | 9 (75) | .550 |
| Female | 3 (37.5) | 3 (25) | – |
| WBC × 109/L | |||
| ≥ 50 | 4 (50) | 9 (75) | .251 |
| < 50b | 4 (50) | 3 (25) | |
| Initial CNS involvement | |||
| Yes | 1 (12.5) | 5 (41.7) | .163 |
| No | 7 (87.5) | 7 (58.3) | |
| Mediastinal mass | |||
| Yes | 1 (12.5) | 3 (25) | .494 |
| No | 7 (87.5) | 9 (75) | |
| Adenopathy | |||
| Yes | 2 (25) | 6 (50) | .264 |
| No | 6 (75) | 6 (50) | |
| Organomegaly | |||
| Yes | 5 (62.5) | 5 (41.7) | .361 |
| No | 3 (37.5) | 7 (58.3) | |
| MRD | |||
| Positive | 2 (25) | 2 (16.7) | .648 |
| Negative | 6 (75) | 10 (83.3) |
Abbreviations: CNS, central nervous system; WBC, white blood cells.
A. The five-year overall survival for 12 children diagnosed with T-cell acute lymphoblastic leukemia after 2010 was significantly higher than in those treated before this year. B. The five-year EFS between the two periods did not reach a statistical difference, however, it was clinically superior for those treated from 2010 onwards.
Eighteen (90 %) patients reached complete CR. Two (10 %) of the 20 patients failed to achieve CR, 1 died during induction and 1 had a transplant without remission.
Hematopoietic stem cell transplant (HSCT)Five (25 %) children received a HSCT, two while in relapse, one in partial and two in complete remission. The HLA-identical (n = 2) and haploidentical (n = 3) transplants were performed. Three patients died due to septic shock (n = 1), gastrointestinal bleeding (n = 1) or pulmonary edema (n = 1). The median follow-up after the HSCT was 19 months (2–22).
RelapseA total of 10 (50 %) patients relapsed, 5 out of 8 (62.5 %) before 2010 and 5 out of 12 (41.6 %) after that year. Relapse occurred at a median of 7.5 months (2–73), with the majority (n = 8, 80 %) of relapses occurring within the first year after diagnosis. Relapsed patients had a 5-year OS of 20 % (CI 95 % 17.59–22.52) vs. 75 % (CI 95 % 72.43–77.37) in patients who did not relapse. (p = .007).
In the whole group, the BM was the main site of relapse; isolated BM relapse (n = 5) was followed by combined BM and CNS (n = 3), isolated CNS (n = 1), and BM and testicular relapse (n = 1). Two children who suffered a relapse in the CNS, one isolated and one combined with the BM, had a CNS infiltration at diagnosis.
Causes of deathTen deaths (50 %) were documented, seven of which before 2010. The main cause was sepsis in four patients (57 %), gastrointestinal bleeding and pulmonary edema in one each, while in one case, further treatment was rejected; after 2010, three children died, the causes being sepsis, acute respiratory failure, and gastrointestinal bleeding. Two patients who died were in remission and eight in relapse.
DiscussionAlthough higher incidence rates of ALL have been reported in Latin American countries, the majority arise from B-lymphoblasts and a small subset, from T-lymphoblasts.12–15 The proportion of T-ALL is lower than the 15.2 % reported in the United States.16 The incidence of T-ALL in our population has been reported at 5 % in children and 8 % in adults,17 in contrast with 10–15% and 25 %, respectively, found in other groups.5,18 Our 5.7 % T-ALL proportion is less than the 12.4 % reported in Mexico City14 and half the 10–11 % reported in other Latin American countries.19–21The lower incidence of T-ALL in our study seems to confirm the geographic variation in the biologic subtype expression due to genetic heterogeneity and diversity according to the ethnic composition.22,23
T-ALL is characterized by a sex distribution favoring males. In our cohort, the male-to-female ratio was 2.3, close to the 2.2 and 3.0 ratios reported in other studies.16,24 The sex distribution ratio is more striking than its B-cell counterpart, which has been reported at around 1.2 in the United States and Latin American countries.16,17 There are studies suggesting the role of specific mutations to explain the male predominance, such as the UTX, which is a sex-specific tumor suppressor protein. 24
Similar to other pediatric T-ALL studies, the median age at diagnosis was 9 years, older than that reported for B-ALL.17,25 Moreover, the females were younger.26 The variation in age between sexes in T-ALL has not been studied in depth, as most epidemiology reports on ALL combine B-ALL and T-ALL data. Importantly, 30 % of our patients had CNS infiltration at diagnosis, compared to 7 % in recent reports.6 This increase in CNS disease can be due to delayed diagnosis, differences in leukemic clone activity, or both.
Remarkably, the OS rate in girls was significantly lower, contrasting with other T-ALL reports, in which boys have a grimmer prognosis.27 In contrast, there was no significant difference in respect to the EFS; this could probably be due to the small study sample. Relapsed T-ALL patients have a dismal outcome; in our cohort most relapses occurred within the first year after diagnosis with a low OS rate of 20 % at 5 years, similar to what has been documented7 and half the 42 % reached for B-cell ALL relapsed children in our own population.28 Importantly, the OS and EFS significantly improved after 2010, reaching 72.2 % and 64.8 %, respectively, not being that far from the 80 % and 75 %, respectively, reported in high-income countries.4 This was most probably a consequence of better treatment and supportive therapy, including opportune administration of prophylactic, as well as therapeutic, antibiotics and antifungals, coupled with on-demand transfusion support with apheresis platelets; platelet support positively influenced outcomes, but before 2010 platelet apheresis for hematology patients was restricted at our public institution due to financial limitations, whereas after this year, it became permanently available. Importantly, regular platelet concentrates at our center are limited due to the reduced percentage of volunteer blood donors, at 2.38 %, compared to 63.62 % in Brazil.29
The improvements in the study period were financed by a federal government program, the “Seguro Popular” (People’s Insurance), that allocates funds for childhood leukemia treatment, as we have previously reported, for the hospitalization of children with ALL.30
The main study limitations include, but are not limited to, the single-center setting and the relatively small sample size.
In conclusion, in our Latin American Hispanic group, the T-cell ALL was a rare leukemia, more frequent in boys, with a worst prognosis in girls, and a high frequency of CNS infiltration; after 2010, intensified chemotherapy, improved blood products support and adequate infection prophylaxis resulted in significantly greater survival rates, approaching those in high-income countries.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Sergio Lozano-Rodriguez, M.D., for his critical review of the manuscript.