To describe the clinical and laboratory features of children and adolescents with acute lymphoblastic leukemia treated at three referral centers in Ceará and evaluate prognostic factors for survival, including age, gender, presenting white blood cell count, immunophenotype, DNA index and early response to treatment.
MethodsSeventy-six under 19-year-old patients with newly diagnosed acute lymphoblastic leukemia treated with the Grupo Brasileiro de Tratamento de Leucemia da Infância – acute lymphoblastic leukemia-93 and -99 protocols between September 2007 and December 2009 were analyzed. The diagnosis was based on cytological, immunophenotypic and cytogenetic criteria. Associations between variables, prognostic factors and response to treatment were analyzed using the chi-square test and Fisher's exact test. Overall and event-free survival were estimated by Kaplan–Meier analysis and compared using the log-rank test. A Cox proportional hazards model was used to identify independent prognostic factors.
ResultsThe average age at diagnosis was 6.3±0.5 years and males were predominant (65%). The most frequently observed clinical features were hepatomegaly, splenomegaly and lymphadenopathy. Central nervous system involvement and mediastinal enlargement occurred in 6.6% and 11.8%, respectively. B-acute lymphoblastic leukemia was more common (89.5%) than T-acute lymphoblastic leukemia. A DNA index >1.16 was found in 19% of patients and was associated with favorable prognosis. On Day 8 of induction therapy, 95% of the patients had lymphoblast counts <1000/μL and white blood cell counts <5.0×109/L. The remission induction rate was 95%, the induction mortality rate was 2.6% and overall survival was 72%.
ConclusionThe prognostic factors identified are compatible with the literature. The 5-year overall and event-free survival rates were lower than those reported for developed countries. As shown by the multivariate analysis, age and baseline white blood cell count were independent prognostic factors.
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children and represents 75–80% of acute leukemia in this age group. The incidence of childhood ALL is 3–4 cases per 100,000 in under 15-year-old children. Despite affecting children of all ages, the incidence peaks between two and five years of age, with a slight predominance among boys.1
ALL is a heterogeneous disease; subtypes differ with regard to biological, cellular and molecular characteristics, response to therapy and risk of relapse, and are associated with different outcomes.2
The survival rate of pediatric ALL patients has improved to approximately 90% in recent years, especially for groups with good prognosis. This progress is mainly due to the adoption of modifications in therapy based on patients’ individual pharmacodynamics and pharmacogenomics, risk-adapted therapy and improved supportive care.2,3
Stratification into risk groups is based on a range of clinical, biological and genetic features, such as age and gender, white blood cell (WBC) count at diagnosis, immunophenotypic, cytogenetic and molecular characteristics, and early medullar response to induction therapy.4–6 Early response to therapy determined by the level of minimal residual disease (MRD) at the end of induction is currently the most important prognostic factor in patients with ALL.7
The identification of prognostic factors, an improved stratification of risk groups and survival analysis have made it possible to identify presenting features of the disease and evaluate treatment outcome in referral centers in Ceará, thereby contributing to current knowledge of the epidemiology of pediatric malignancies, both locally and throughout all Brazil.
The purpose of this study was to describe the clinical and laboratory features of children and adolescents with ALL treated at three referral centers in the state of Ceará, Brazil and evaluate prognostic factors for survival, including age, gender, presenting WBC count, immunophenotype, DNA index and early response to treatment.
MethodsThis prospective study was based on a sample of 76 under 19-year-old patients with newly diagnosed ALL treated with the Grupo Brasileiro de Tratamento de Leucemia da Infância – acute lymphoblastic leukemia (GBTLI-ALL)-93 protocol (n=64) or the GBTLI-ALL-99 protocol (n=12) between September 2007 and December 2009. Patients were treated at the pediatric oncology services of Hospital Infantil Albert Sabin (HIAS), Hospital do Câncer Haroldo Juaçaba (HC) and Hospital Luis França (HLF). HIAS and HC are public referral hospitals providing treatment of childhood cancer in Ceará. All parents and/or patients gave their written informed consent. The study was approved by the Research Ethics Committees of HC (Protocol 66/2007) and HIAS (Protocol 058/08).
The observed clinical variables included age at diagnosis, gender, hepatomegaly, splenomegaly, lymphadenopathy, mediastinal mass, infiltration of the central nervous system (CNS), WBC count, hemoglobin and lactate dehydrogenase (LDH) levels, platelet count, immunophenotype and DNA Index (DI).
Treatment response was evaluated based on the presenting WBC count, the lymphoblast count on the eighth day of induction therapy (Day 8), and bone marrow (BM) analysis on Day 28 of induction.
ALL was diagnosed in patients with ≥25% lymphoblasts in BM (based on morphological and cytochemical evaluations of BM smears) and positivity in immunophenotyping and cytogenetics. The DI was determined by flow cytometry. Peripheral blood, bone marrow and cerebrospinal fluid (CSF) were collected at referral hospitals.
CNS involvement was diagnosed in patients with >5 WBC/μL in CSF and lymphoblasts identified on the cytocentrifuge slide.
According to the GBTLI risk criteria, risk of relapse (low vs. high) was stratified based on age, WBC count at diagnosis and early response to therapy. The following pretreatment characteristics were observed in high-risk patients: age less than one, age greater than nine, WBC >50.0×109cells/L, and lymphoblast count >1000cells/μL or WBC count >5.0×109cells/L on Day 8. All other patients were classified as low risk of relapse.8,9
Statistical analysisThe statistical analysis was carried out using the R statistical language version 2.1.10 Descriptive statistics were used to characterize the patients. Associations between variables, prognostic factors and response were analyzed with the chi-square test and Fisher's exact test. Five-year overall survival (OS) and event-free survival (EFS) rates were estimated with the Kaplan–Meier method and compared with the log-rank test. The Cox proportional hazards model11 was used to identify independent prognostic factors with respect to EFS and OS. The level of statistical significance was set at 5% (p-value <0.05).
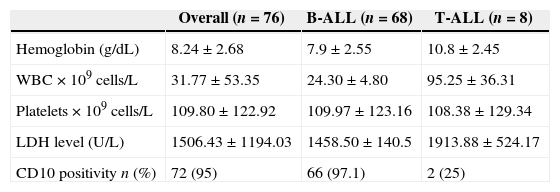
ResultsThe clinical and laboratory features at diagnosis for this sample of 76 patients are summarized in Tables 1 and 2. Low and high risk of relapse was attributed to 54% (n=41) and 46% (n=35) of the sample, respectively.
Presenting clinical and biological characteristics of 76 children and adolescents with acute lymphoblastic leukemia (B-ALL and T-ALL) treated at three referral centers in Ceará, Brazil, between September 2007 and December 2009.
| Overall (n=76) | B-ALL (n=68) | T-ALL (n=8) | |
|---|---|---|---|
| Age (years) | |||
| <1 | 2 (2.6) | 2 (2.94) | – |
| ≥1<9 | 57 (75) | 54 (79.4) | 3 (37.5) |
| ≥9 | 17 (22.4) | 12 (17.6) | 5 (62.5) |
| Gender | |||
| Male | 50 (65.8) | 44 (64.7) | 6 (75) |
| Female | 26 (34.2) | 24 (35.3) | 2 (25) |
| Risk group | |||
| High | 35 (46) | 28 (41.2) | 7 (87.5) |
| Low | 41 (54) | 40 (58.8) | 1 (12.5) |
| FAB | |||
| L1 | 63 (83) | 56 (82.4) | 7 (87.5) |
| L2 | 13 (17) | 12 (17.6) | 1 (12.5) |
| Extramedullary invasion | |||
| Hepatomegaly | 48 (63) | 43 (63.2) | 5 (62.5) |
| Splenomegaly | 44 (57.8) | 40 (58.8) | 4 (50) |
| Lymphadenopathy | 33 (43.4) | 26 (38.2) | 7 (87.5) |
| CNS disease | 5 (6.5) | 4 (5.9) | 1 (12.5) |
| Mediastinal mass | 9 (11.8) | 3 (4.4) | 6 (75) |
Values are expressed in absolute numbers of patients with certain characteristics (percentage relative to the total number of patients in their subgroup in parentheses). FAB: French-American-British; CNS: central nervous system.
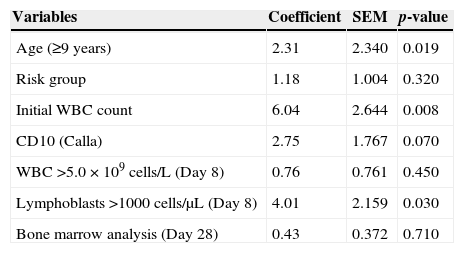
Baseline laboratory characteristics of 76 children and adolescents with acute lymphoblastic leukemia (B-ALL and T-ALL) treated at three referral centers in Ceará, Brazil, between September 2007 and December 2009.
| Overall (n=76) | B-ALL (n=68) | T-ALL (n=8) | |
|---|---|---|---|
| Hemoglobin (g/dL) | 8.24±2.68 | 7.9±2.55 | 10.8±2.45 |
| WBC×109cells/L | 31.77±53.35 | 24.30±4.80 | 95.25±36.31 |
| Platelets×109cells/L | 109.80±122.92 | 109.97±123.16 | 108.38±129.34 |
| LDH level (U/L) | 1506.43±1194.03 | 1458.50±140.5 | 1913.88±524.17 |
| CD10 positivity n (%) | 72 (95) | 66 (97.1) | 2 (25) |
Levels expressed as mean values±standard error of the mean (SEM).
WBC: white blood cells; LDH: lactate dehydrogenase.
The average age at diagnosis was 6.3±0.5 years (median=5.3). Males (65.8%) were predominant in the sample (male/female ratio=1.9:1).
The most prevalent age group was one to nine years (75%). Under one-year-old and over nine-year-old patients accounted for 2.6% and 22.4%, respectively. Age less than one was associated with unfavorable prognostic factors, such as presenting a high baseline WBC count, pro-B immunophenotype and CD10 negativity. Age between one and nine years was associated with more favorable prognostic factors, such as a WBC count <50.0×109cells/L, CD10 positivity and DI >1.16.
The most frequent clinical features were hepatomegaly, splenomegaly, fever and lymphadenopathy. CNS involvement and mediastinal mass were observed in 6.6% and 11.8%, respectively.
Anemia was diagnosed in 85% of the patients, 35% of whom had severe anemia (Hb<7g/dL); the mean hemoglobin level was 8.24g/dL. At diagnosis, patients with T-ALL presented significantly higher Hb levels (10.6±2.45g/dL) than patients with B-ALL (7.9±2.55g/dL; p-value=0.011). A platelet count <100.0×109cells/L was observed in 65%, and 10.5% had severe thrombocytopenia with platelet counts <20.0×109cells/L. The average WBC count at diagnosis was 31.8±53.4×109cells/L (range: 0.9–320.0×109cells/L). Sixteen patients (21%) were classified as high risk of relapse (WBC count >50.0×109cells/L) (Table 2). In 4%, the peripheral blood cell count was normal at diagnosis, with no changes in hemoglobin level, WBC or platelet counts and no lymphoblasts in peripheral blood smears.
LDH >1000U/L was used as a prognostic marker (normal: 480U/L). Thus, 39 patients (51.3%) presented LDH levels >1000U/L (mean: 1506.43±1194.03U/L) (Table 2).
B immunophenotype ALL was identified in 89.5%. The most prevalent subtypes were common B-ALL (51.5%) and pre-B (45.5%). Pro-B was found in about 3% of cases. Patients with mature B or French-American-British (FAB) classification of L3 were not included in this study.
T immunophenotype ALL was found in 10.5%. The most prevalent European Group for the Immunological Characterization of Leukemias (EGIL) subtypes were pre-T (50%) and cortical T (37.5%). T-ALL was associated with unfavorable factors including the following: being male, over nine years in age, leukocytosis, CD10 negativity, mediastinal mass, and CNS involvement (Table 1).
DI was determined by flow cytometry in 58 patients. Overall, 76% were diploid (DI=1) and 24% were hyperdiploid (DI>1.0). Observed in 19%, DI >1.16 was associated with low risk of relapse and favorable factors such as age between one and nine years, WBC count <20.0×109cells/L (p-value=0.028), B immunophenotype, and 100% CD10 positivity (Table 3). These patients responded well to induction therapy (on Day 8, lymphoblast count <1000/μL and WBC count <5.0×109cells/L), with complete hematological remission and absence of early relapse. Outcome according to DI showed that patients with DI >1.16 fared better (5-year EFS: 100%) than patients with DI <1.16 (5-year EFS: 66%; p-value=0.03).
Characteristics according to DNA ploidy of 76 children and adolescents with acute lymphoblastic leukemia treated at three referral centers in Ceará, Brazil, between September 2007 and December 2009.
| DI<1.16 (n=47) | DI>1.16 (n=11) | p-value | |
|---|---|---|---|
| Age – n (%) | |||
| 1<5 years | 24 (51) | 7 (63.6) | 0.309 |
| 5<9 years | 14 (30) | 4 (36.4) | |
| ≥9 years | 9 (19) | – | |
| Risk group – n (%) | |||
| Low | 25 (53.2) | 10 (91) | 0.037 |
| High | 22 (46.8) | 1 (9.0) | |
| Immunophenotype – n (%) | |||
| B | 44 (93.6) | 11 (100) | 1.000 |
| T | 3 (54) | – | |
| Initial WBC count – n (%) | |||
| <20.0×109cells/L | 27 (57.5) | 11 (100) | 0.028 |
| >20.0–50.0×109cells/L | 8 (17) | – | |
| >50.0×109cells/L | 12 (25.5) | – | |
| CD10 – n (%) | |||
| Positive | 42 (89.4) | 11 (100) | 0.572 |
| Negative | 5 (10.6) | – | |
Values are expressed in absolute numbers of patients with certain characteristics (percentage relative to the total number of patients in their subgroup in parentheses).
DI: DNA Index.
The median follow-up was 60 months. Seventy-two patients (95%) achieved complete remission. On Day 8, 95% of the patients had a lymphoblast count <1000/μL and WBC count <5.0×109cells/L. Twelve (16%) patients relapsed and two (2.6%) died during induction.
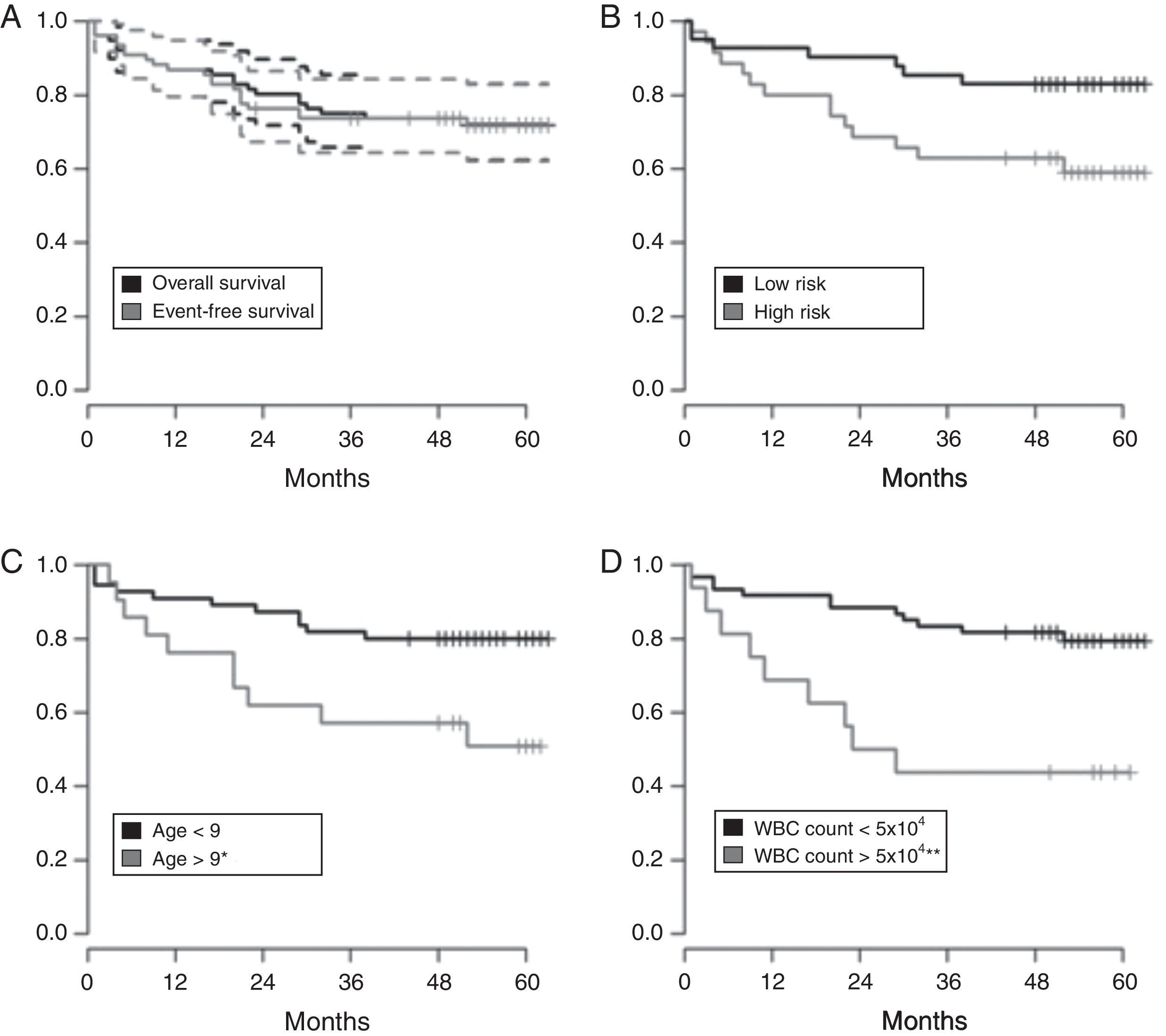
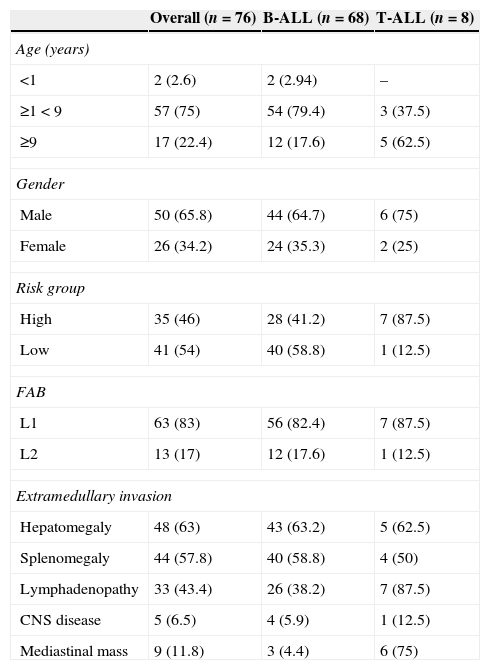
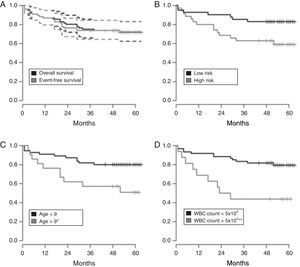
EFS for all 76 patients was 71.2±5.2% [95% confidence interval (95% CI): 62.1–82.8%] and 5-year OS was 72±5.24% (95% CI: 62–82%), with higher EFS rates in the low-risk group (83%; 95% CI: 72–95%) and in patients with B-ALL (73%; 95% CI: 63–85%) (Figure 1A and B).
Kaplan–Meyer estimate of overall survival (OS) and event-free survival (EFS) of children and adolescents with acute lymphoblastic leukemia. A – OS in Black and EFS in gray, with 95% confidence interval; B – OS according to risk group (low and high risk); C – OS according to age at diagnosis less than or greater than nine years old); D – OS based on white blood cell count at diagnosis (less than or greater than 50×103/mm3). Median follow-up was 60 months. Statistical significance in the multivariate analysis: *p-value <0.05; **p-value <0.01.
Age at diagnosis and presenting WBC count were statistically significant prognostic factors. The highest EFS rates were observed in children aged one to nine years (73%; 95% CI: 63–85%) and in patients with presenting WBC count <50.0×109cells/L (73%; 95% CI: 63–85%) (Figure 1C and D).
In the multivariate analysis, age at diagnosis, presenting WBC count and lymphoblast count on Day 8 remained statistically significant with regard to risk of relapse after adjusting for gender, risk group, CD10 expression, and early response to therapy (Table 3).
The comparison of relative risk revealed that the risk was higher for patients with WBC counts >50.0×109cells/L at diagnosis (HR=589; 95% CI: 7–4757). The wide range of the CI is due to the relatively small number of patients with elevated WBC counts (n=16). The other prognostic factors (age and number of lymphoblasts on Day 8) had a relative risk between 0.2 and 1.0, with a wide variation of 95% CI, indicating a weaker influence on OS. The same prognostic factors were associated with EFS, with relative risks of the same magnitude.
DiscussionLittle has been published on the epidemiology of childhood and juvenile ALL in Northeastern Brazil. The present study looks into the epidemiological and clinical aspects, prognostic factors and response to therapy of children with ALL treated at referral centers in the state of Ceará.
The age of our patients was significantly correlated with prognosis. Thus, under 1-year-old infants and patients older than nine presented poorer prognoses. Infancy was associated with unfavorable prognostic factors such as high presenting WBC count, Pro-B immunophenotype, CD10 negativity, hepatosplenomegaly and poor response to induction treatment, thus matching findings in the literature.12
Over nine-year old children and adolescents accounted for 22.4% of the cases in this study. Historically, adolescents and young adults have higher relapse rates and shorter survival than children aged one to nine years. The worse outcomes in this group may be due to the high prevalence of biologically high-risk leukemia (e.g. breakpoint cluster region protein-Abelson murine leukemia viral oncogene homolog 1 [BCR-ABL1] and mixed-lineage leukemia [MLL] rearrangements) in association with unfavorable factors such as high WBC count, T-cell immunophenotype, and poor adherence to and tolerance of therapy.13,14
Recent studies have shown that adolescents and young adults have better outcomes and higher rates of continuous remission when treated according to pediatric protocols with higher doses of non-myelosuppressive drugs, rather than with adult regimens.14,15
Males were predominant in the present study matching the findings of Brazilian studies from Bahia,16 Pernambuco,17 and Rio Grande do Sul,18 and the GBTLI-ALL-99 protocol.19 In two studies, males with low EFS rates had unfavorable prognoses.19,20
Hepatomegaly, splenomegaly and lymphadenopathy, fever and severe anemia were the most frequently observed clinical features in this study as in other published reports.1 CNS involvement occurred in 6.6% of the patients. This is consistent with the rate (6.7%) reported in Bahia,16 and elsewhere in the world (0.6–12.4%).21 The GBTLI-ALL 93 protocol and the GBTLI-ALL 99 protocol yielded lower rates (1.7% and 2.4%, respectively).8,9
Anemia occurred in 85% of the patients in the current study, most of whom were normochromic and normocytic. Severe anemia (Hb <7g/dL) was observed in 35%. Patients with T-ALL presented significantly higher Hb levels at diagnosis than patients with B-ALL (mean Hb level, 10.6g/dL vs. 7.9g/dL). According to some authors, lower Hb levels at diagnosis are associated with more advanced disease.22
Platelet counts <20.0×109cells/L were found in 10.5%. Despite the increased risk of bleeding; no severe bleeding was observed in our sample.
WBC count at diagnosis >50.0×109cells/L was registered in 21% of the patients, matching international studies that report WBC counts >50.0×109cells/L in approximately 20% of cases.21 Patients with severe leukocytosis at diagnosis may present bulky tumor mass, mediastinal enlargement, hepatosplenomegaly and significant lymphadenopathy. This finding is usually associated with the unfavorable chromosomal translocations t(4;11) and t(9;22).
T-ALL was observed in 10.5%. This incidence is slightly lower than those of the GBTLI-ALL 93 and GBTLI-ALL 99 protocols (13% and 14.2%, respectively)8,9 but consistent with other national and international studies (7.4–16.4%).21,23
In this study, patients with T-ALL were more likely to present unfavorable factors such as being male, age greater than nine years, high WBC count at diagnosis, low CD10 expression, mediastinal enlargement and CNS involvement (Tables 1 and 2). These results are compatible with those reported by Goldberg et al. 23
Historically, the T-cell immunophenotype has been considered an adverse clinical prognostic factor in childhood ALL, although its effect has been reduced by contemporary risk-adapted therapy and improved supportive care.23 Nevertheless, patients with this immunophenotype are still at increased risk of induction failure, early relapse and isolated CNS relapse.
In the present study, DI >1.16 was associated with a favorable prognosis (Table 3). In fact, at the end of induction, all patients with DI >1.16 were alive and were in complete remission with no early recurrence. These findings are compatible with the results of Aricó et al.24 and Dastugue et al.,25 who found a strong association between hyperdiploidy and favorable prognostic factors such as age between one and five years and WBC count at diagnosis <20.0×109cells/L.
In one study,24 the OS was 95–96% and EFS was 89% in patients with DI ≥1.16 (corresponding to 53 chromosomes). More recent investigations have shown that patients with B-ALL hyperdiploidy (58–66 chromosomes) are almost completely curable.25 In contrast, hypodiploid patients with fewer than 45 chromosomes (no more than 2% of cases) have a poor prognosis.
Early response to treatment was defined as a reduction in peripheral WBCs and circulating lymphoblasts on Day 8 and early bone marrow response at the end of induction (Day 28).26,27
On Day 8, 5% of our patients had lymphoblast counts >1000cells/μL and WBC counts >5.0×109cells/L. Defined as poor responders, these patients were associated with significantly lower EFS rates. Poor responders also displayed lower EFS rates (∼45–52%) in the GBTLI-ALL-99 protocol.9 According to Manabe et al., patients with no lymphoblasts in the peripheral blood on Day 8 had an excellent outcome, with over 90% surviving at four years.28 A recent study by Vaghela et al. supports the prognostic value of peripheral lymphoblast counts on Day 8 of induction therapy.29
At the end of induction, 95% of the patients in the current study had achieved remission. This is in agreement with Brazilian and international studies that show a continuous remission rate of 95–99%.9,20 In this study, the bone marrow evaluation on Day 28 was performed only by morphological analysis, although many authors have stressed the significance of response to remission induction therapy expressed by minimal residual disease (MRD).7 In 30% of patients treated according to the Brazilian GBTLI LLA-99 study, the presence of MRD on Day 28 of induction was the independent prognostic factor with the greatest impact in multivariate analysis, when the EFS was analyzed in relation to the age and WBC count at diagnosis.9
In the GBTLI-ALL-2009 study, MRD was used to identify high-risk patients and use of intensive induction therapy. The allocation of these patients according to “molecular response” to treatment and early intensification of therapy may improve the outcome in childhood ALL in the state of Ceará and Brazil.
Death during induction therapy occurred in 2.6% of the cases in this study. This is similar to the results of the GBTLI-ALL-93 protocol (3%), but higher than the rates reported for developed countries (0.5–1.4%),21,23 suggesting the induction mortality rate is short of satisfactory at Brazilian centers. In this study, infection was the most common cause of death.
At five years, the OS and EFS in the current sample were 72% and 71.2%, respectively. As expected, the survival rate was higher for low-risk patients (83%) than for high-risk patients (59%; Figure 1A and B). The corresponding figures were 70%±3.6% and 69%±2.4% for the GBTLI-ALL-93 protocol and 74.2±1.7% and 68±1.8% for the GBTLI-ALL-99 protocol, with a significant difference in favor of low-risk patients.8,9 Our figures are slightly better than those of Leite et al.17 and Pereira,18 but not as good as the figures currently reported by American and European collaborative childhood cancer treatment groups.21,23
The OS and EFS were better for B-ALL patients, though the difference was not statistically significant. This finding differs from the GBTLI-ALL-93 protocol in which the EFS at six years was significantly lower for T-ALL patients.8 This disagreement might be attributed to the small number of T-ALL patients in our study; however, in a study published by the Dana-Farber group, differences in survival between T-ALL and B-ALL (78%±4% vs. 86%±1%) were not significant either, despite the finding of increased risk of induction failure and relapse.23
In the current study, the OS was significantly better for under nine-year-old patients and patients with WBC count at diagnosis <50.0×109cells/L (Figure 1C and 1D), as documented in several other studies.8,9,21,23
In the multivariate analysis (Table 4), WBC count at diagnosis >50.0×109cells/L, age greater than nine years and high peripheral lymphoblast counts (>1000cells/μL) on Day 8 were significantly associated with poor survival. No other prognostic factors (FAB classification, CD10 expression or DI) were significant.
Results of the Cox proportional hazards model of prognostic factors of acute lymphoblastic leukemia for a sample of 76 children and adolescents treated at three referral centers in Ceará, Brazil, between September 2007 and December 2009.
| Variables | Coefficient | SEM | p-value |
|---|---|---|---|
| Age (≥9 years) | 2.31 | 2.340 | 0.019 |
| Risk group | 1.18 | 1.004 | 0.320 |
| Initial WBC count | 6.04 | 2.644 | 0.008 |
| CD10 (Calla) | 2.75 | 1.767 | 0.070 |
| WBC >5.0×109cells/L (Day 8) | 0.76 | 0.761 | 0.450 |
| Lymphoblasts >1000cells/μL (Day 8) | 4.01 | 2.159 | 0.030 |
| Bone marrow analysis (Day 28) | 0.43 | 0.372 | 0.710 |
SEM: standard error of the mean; WBC: white blood cell count.
Overall, the clinical and laboratory features observed in this study are compatible with the results reported in the GBTLI-ALL 93 and 99 protocols and elsewhere in the literature. The multivariate analysis indicated that age, initial WBC count and early response to treatment were independent prognostic factors, whereas gender, FAB classification, CD10 expression and DI were not.
The estimated survival rates found were inferior to the results obtained in developed countries. The 5-year EFS rate was lower than that reported in the literature. However, low-risk patients had a better prognosis.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to express our thanks to Dr. Antônio Aldo Melo and Dr.a Maria Helena Pitombeira from the Federal University of Ceará for their critical review of the manuscript.