Detecting anti-PF4 antibodies remains the golden diagnostic method for heparin-induced thrombocytopenia (HIT) diagnosis with high sensitivity and specificity. Various lab tests detect anti-PF4 antibodies, including immunoassays and functional assays. Even with positive detection of the anti-PF4 antibody, several factors are involved in the result. The concept of anti-PF4 disorders was recently brought to light during the COVID pandemic since the development of vaccine-induced thrombotic thrombocytopenia (VITT) with the adenovirus-vectored-DNA vaccine during the pandemic. Circumstances that detect anti-PF4 antibodies are classified as anti-PF4 disorders, including VITT, autoimmune HIT and spontaneous HIT. Some studies showed a higher percentage of anti-PF4 antibody detection among the population infected by COVID-19 without heparin exposure and some supported the theory that the anti-PF4 antibodies were related to the disease severity. In this review article, we provide a brief review of anti-PF4 disorders and summarize the current studies of anti-PF4 antibodies and COVID-19 infection.
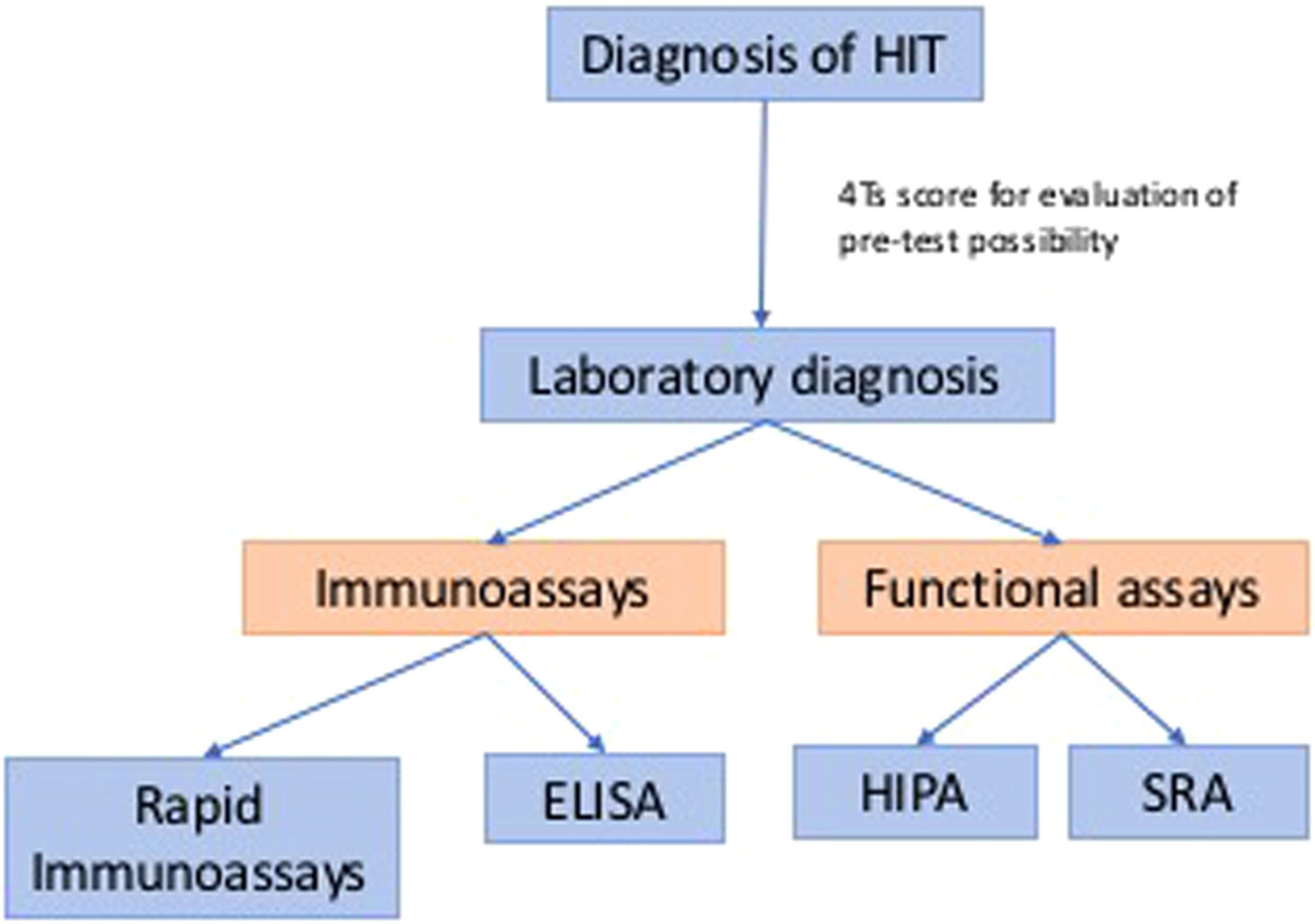
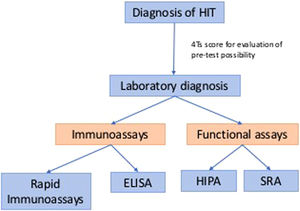
Traditional diagnosis of classic HIT usually involves two parts: clinical and laboratory diagnoses. Clinical history can help decide the pre-test possibility before we pursue laboratory diagnosis, such as the 4Ts score. Laboratory tests remain the golden criterion for the detection of HIT. Laboratory diagnoses are summarized in Figure 1, which includes immunoassays and functional assays.1 The theory of the “iceberg model” was introduced by Dr. Warkentin, which illustrated functional assays (platelet activation assays), including the serotonin-release assay (SRA) and the heparin-induced platelet activation (HIPA) assay, which have similar high sensitivity as PF4-dependent immunoassays, such as enzyme immunoassays (EIAs), but higher specificity. This concept also showed that SRA and HIPA are both suitable for detecting heparin-independent platelet-activating antibodies, which are essential in diagnosing anti-PF4 disorders.2
Anti-PF4 disorders are new concepts evolving after the COVID-19 pandemic, which included the classic HIT, autoimmune HIT, spontaneous HIT and VITT, introduced by Dr. Warkentin. Some patients still developed anti-PF4 antibodies without exposure to heparin and, interestingly, compared to the traditional enzyme-linked immunoassay (ELISA), rapid immunoassays showed less sensitivity in detecting VITTs. Research with the detection of anti-PF4 antibodies in patients with COVID-19 usually used the ELISA, a standard method for diagnosis, as its method of choice. Compared to most studies on VITT, fewer studies mentioned anti-PF4 antibody levels in patients with COVID-19 infection without heparin exposure. Some research mentioned the detection of anti-PF4 antibodies in patients with COVID-19 without exposure to heparin-related products, which might be related to the disease severity. In the following sections, we will review anti-PF4 disorders and summarize the current relationship between anti-PF4 antibodies and the COVID-19 infection.
Anti-PF4 disordersExcept for the patients with previous heparin exposure, some patients with no history of heparin exposure were found to have anti-PF4 antibodies. With the advancement of technology, the evolution of vaccine-induced thrombotic thrombocytopenia (VITT) with the use of the adenovirus-vectored-DNA vaccine during the pandemic became noteworthy. The concept of anti-PF4 disorders was introduced, which included classic HIT, autoimmune HIT, spontaneous HIT and VITT.3
These four categories share some common characteristics, including pan-cellular activation, which means not only the platelets are involved in the pathophysiology process, but also other cells, such as monocytes and polymorphonuclear leukocytes (PMNs) and the classic complement pathway. Higher plasma myeloperoxidase (MPO) concentrations within HIT patients also indicated that leukocyte degranulation is involved.4 Complement activation was observed; anti-C1q antibodies can prevent complement activation by PF4/heparin complexes, which indicated the involvement of the classic complement pathway.5
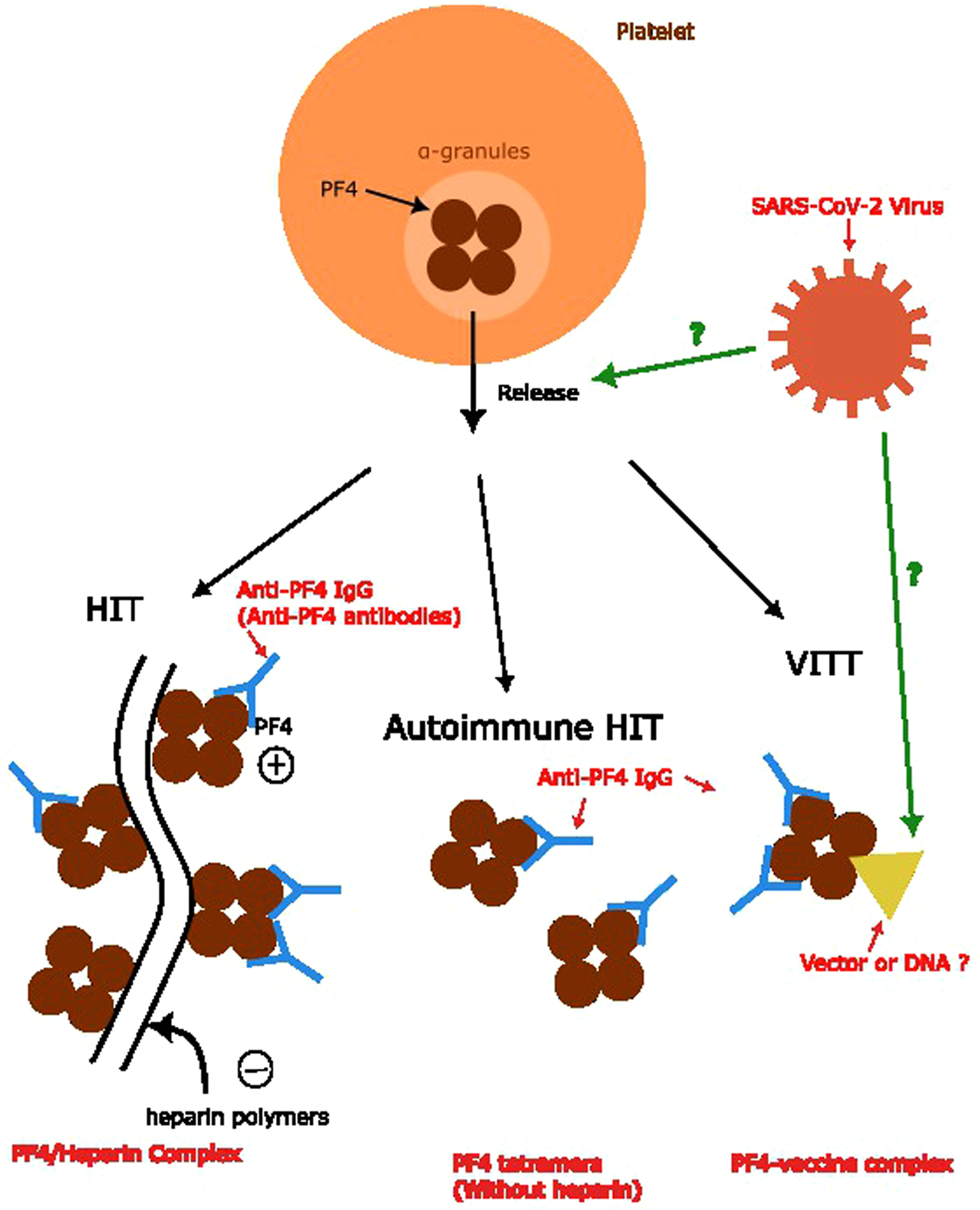
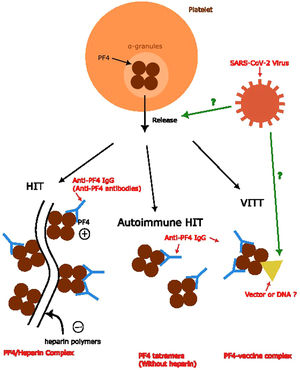
According to the etiology of the PF4 complex formation in various anti-PF4 disorders, the complex trigger anti-PF4 antibodies are different. In classic HIT, the PF4 combines with the heparin polymers with a negative charge to form a PF4/heparin complex. In the autoimmune HIT, this process does not involve heparin. The mechanism of VITT, theoretically, is more likely to involve either vector or DNA.6 However, the relationship between anti-PF4 antibodies and the infection of COVID remains unknown, as well as the mechanism of the formation of anti-PF4 antibodies in patients infected by the SARS-CoV-2 virus. Several different models have been proposed. One proposal is similar to the formation of PF4-vaccine complexes, which includes the part of the virus involved in the formation of the complex. Another one suggests the facilitation of the release of PF4s from platelets induces a mechanism similar to autoimmune HIT.6,7 In summary, we conclude the mechanism of anti-PF4 antibody formation in Figure 2.
Anti-PF4 antibody formation and relationship with anti-PF4 disorders. Anti-PF4 antibodies are the main concepts for anti-PF4 disorders. Formation of the PF4/heparin complex and PF4 tetramers without combining heparin polymers leads to anti-PF4 antibody formation in the classic HIT and autoimmune HIT, respectively. The theory behind the formation of anti-PF4 antibodies in VITT and its relationship with SARS-CoV-2 viruses remains unknown, which we used question marks to represent.6,7
The autoimmune HIT involves heparin-independent platelet activation induced by aHIT antibodies, which can activate platelets without heparin and bind to PF4.6,8 Polyphosphate/PF4 complexes released by activated platelets can mediate platelet aggregation by anti-PF4 antibodies without heparin or cell-surface chondroitin sulfate,9 which is likely the mechanism behind the presence of both heparin-dependent and heparin-independent platelet-activating antibodies in patients with aHIT,10 Heparin-independent anti-PF4 antibodies are probably facilitated by non-heparin platelet-associated polyanions (chondroitin sulfate and polyphosphates).8
According to clinical situations, there are different sub-classifications within aHIT, including refractory HIT, delayed-onset HIT, fondaparinux-associated HIT and heparin “flush” HIT. Several published case reports are related to delayed-onset HIT and clinically delayed-onset heparin is also associated with arterial and venous thrombosis.11,12 On the other hand, refractory HIT indicates thrombocytopenia persists despite stopping heparin, which can last up to weeks.13
Heparin “flush” HIT indicates a small amount of heparin, such as heparin flushes and triggers HIT, which also involves the heparin-independent platelet-activating antibodies. In the study by Dr. Mian, four patients reporting HIT induced by heparin flush showed strong heparin-independent platelet activation.14 In Kadidal's review, cases of HIT from 1968 to 1998 were reviewed from the MEDLINE database, concluding that only 29 cases were attributed to heparin flushes.15 The fondaparinux-associated HIT is HIT caused by fondaparinux, a synthetic pentasaccharide that shares a sequence of five monomeric sugar units with heparin.16 However, the concept of pseudo-HIT was mentioned, which may suggest that some cases of fondaparinux-associated HIT are not truly caused by fondaparinux, but by other reasons that have not been revealed.17
Spontaneous HITThere were several circumstances that anti-PF4 antibodies were detected in patients without heparin-related exposure, but there was no evidence showing those antibodies have platelet-activating features. Still, in Warkentin's case report, three patients had high levels of anti-PF4–heparin antibodies with strong platelet-activating properties in the absence of heparin exposure, but with preceding acute infectious, inflammatory events or after orthopedic surgery.18,19 There are additional cases reported after 2008 and most can be classified into two different categories, which are surgical spontaneous HITs and medical spontaneous HITs, according to different etiologies. Orthopedic surgeries, especially total knee arthroplasty, are the most common cause of spontaneous HIT. The adrenal hemorrhage or necrosis secondary to adrenal vein thrombosis also has a high frequency in surgical spontaneous HIT, but both venous and arterial thrombosis have been observed.3,20 Higher incidences of cerebral venous thrombosis and arterial stroke were found in medical spontaneous HIT caused by acute infectious and/or inflammatory events.3,20
Vaccine-induced immune thrombotic thrombocytopenia (VITT)The VITT is a newly identified classification of thrombocytopenia found after the development of the ChAdOx1 nCov-19 vaccination in 2019, when five patients were found to develop venous thrombosis and thrombocytopenia after receiving adenoviral vector vaccines against coronavirus disease 2019 (Covid-19).21 Similar to the ChAdOx1 nCov-19 vaccination (AstraZeneca), the patients receiving the Ad26. COV2.S vaccine (Janssen; Johnson & Johnson) also presented several reported cases of VITT.22 Compared to adenoviral vector vaccines, messenger RNA (mRNA) technology-based vaccines against SARS-CoV-2 are generally unrelated to VITT.23 However, a case reported in 2021 met the criteria for VITT after the patient received the messenger RNA–1273 vaccine and had a positive PF4 test result from the ELISA24. The term “thrombosis with thrombocytopenia syndrome” is also used to represent the VITT in the CDC reports. Most cases happened among women aged less than 50 years old.25
The pathologic mechanism of the VITT, which is also induced by anti-PF4 antibodies,26 causes pan-cellular activation, similar to the mechanism we mentioned before in other anti-PF4 disorders, similar to the spontaneous HIT. The 2-step mechanism was mentioned in the study of Greinacher et al. through mouse models, which included the formation of PF4/vaccine complex and the B-cell response triggered by vaccine-induced inflammation that results in the formation of high-avidity anti-PF4 antibodies.27 The protein components causing the formation of PF4/vaccine complexes remain uncertain, with one of the theories being the free DNA in the vaccines.28 Another theory is that viral vectors can form a complex with PF4, confirmed by a stimulation performed by Baker et al.29
Clinically, cases of VITT demonstrated a similar clinical picture as the spontaneous HIT, which included unusual cerebral venous thrombosis and splanchnic vein thrombosis, usually about 5 to 20 days after vaccination.26,28 The presence of thrombocytopenia, thrombosis, a very high d-dimer level and a low or average fibrinogen level can also be found in patients with VITT.26 Like other anti-PF4 disorders, the VITT involves arterial thrombosis, but sometimes also involves both arterial and venous thrombosis.30 The treatment for the VITT typically involves non-heparin anticoagulants and IVIGs. Heparin or heparin-related products are considered contraindications due to the hypothesis of the cross-reactivity of platelet-activating antibodies against PF4.31-33 As a result, parenteral direct thrombin inhibitors, such as argatroban or bivalirudin, are considered first-line therapy instead, followed by direct oral anti-coagulants (DOACs), such as apixaban or rivaroxaban, or synthetic inhibitors of factor Xa as alternatives.34 Similar to the classic HIT, avoidance of vitamin K antagonists, such as warfarin, is recommended in VITT patients due to the prothrombotic nature of the VITT. Warfarin's early effects on protein C reduction can precipitate venous limb gangrene and/or skin necrosis in patients with the HIT at an incidence rate of more than 5 %.35-37
The usual diagnostic method for the VITT is the ELISA, followed by the confirmation with the functional heparin-induced platelet activation assay (HIPA) or the serotonin-release assay (SRA).26,38 The reason behind this agorism is the high sensitivity of ELISA, which leads to a low false negative rate. According to current data, the ELISA HIT assays are highly sensitive to all anti-PF4 disorders, including the HIT and VITT.3,26,39 In Sachs's study, five different immunoassays were used. They received results of more than 92 % detection of the VITT using the ELISA, 25 % positive through the particle gel immunoassay (PaGIA) and only 8 % showed borderline results through the lateral flow assay (LFA). All cases examined by the IgG-chemiluminescence immunoassay (CLIA) showed non-reactive.40 In contrast, the CLIA showed a sensitivity of 98.8 % and a specificity of 98.5 % in HIT-positive patients, which is significantly higher than in patients diagnosed as VITTs41 and leads to the thought that the presence of additional polar groups on polystyrol microtiter plates used for the ELISA and the availability of PF4 in higher amounts might be the reasons behind this phenomenon.40 Platton's study evaluated four IgG-specific ELISAs and two polyspecific ELISAs. The conclusion was that IgG-ELISAs and polyspecific ELISAs have a similar sensitivity. No single ELISA method detected all VITT cases, which means the second ELISA test should be introduced to diagnose the VITT.42 Based on the above conclusion, rapid HIT assays should be avoided as a diagnosis method for the VITT.
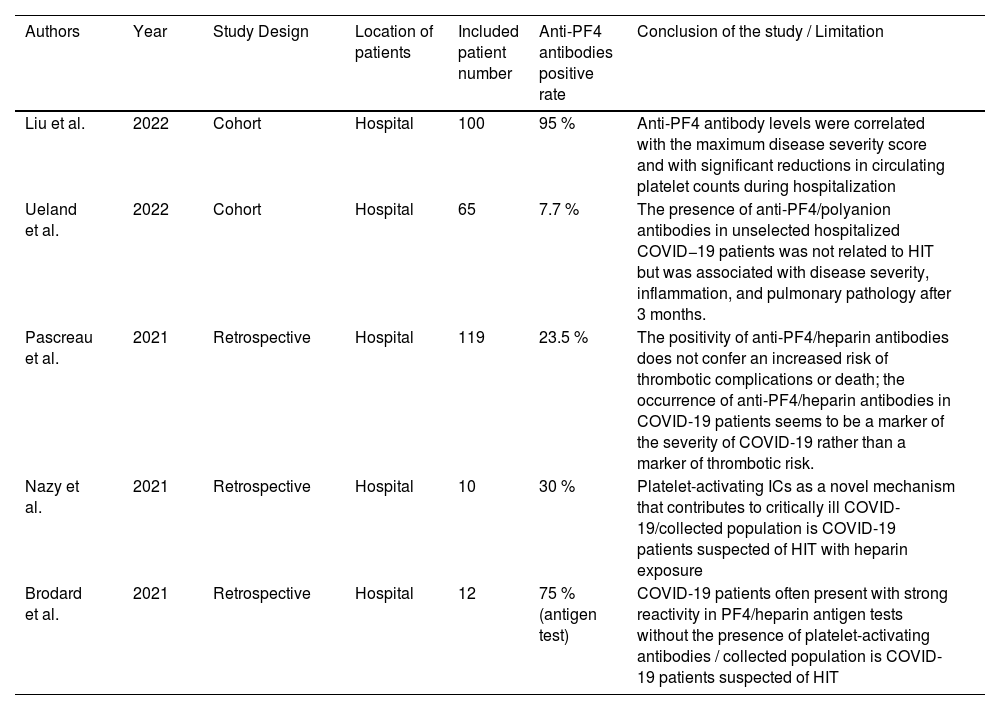
COVID and PF4 antibodiesDisseminated micro-thrombosis and disseminated platelet activation are features of patients with severe coronavirus disease 2019 (COVID-19). Thrombotic events in COVID patients were found during the pandemic, which brought the discussion of the COVID-19-associated hypercoagulopathy to the table. Acute phase reactants and endothelial dysfunction are hypothesized to contribute to this phenomenon.43 Compared to the discussion of the VITT, the discussion of the connection between COVID-19 and PF4 antibodies is not common. In this article, we summarize the currently published articles related to this topic and list them in Table 1.
Summary of articles related to anti-PF4 antibodies and COVID-19.
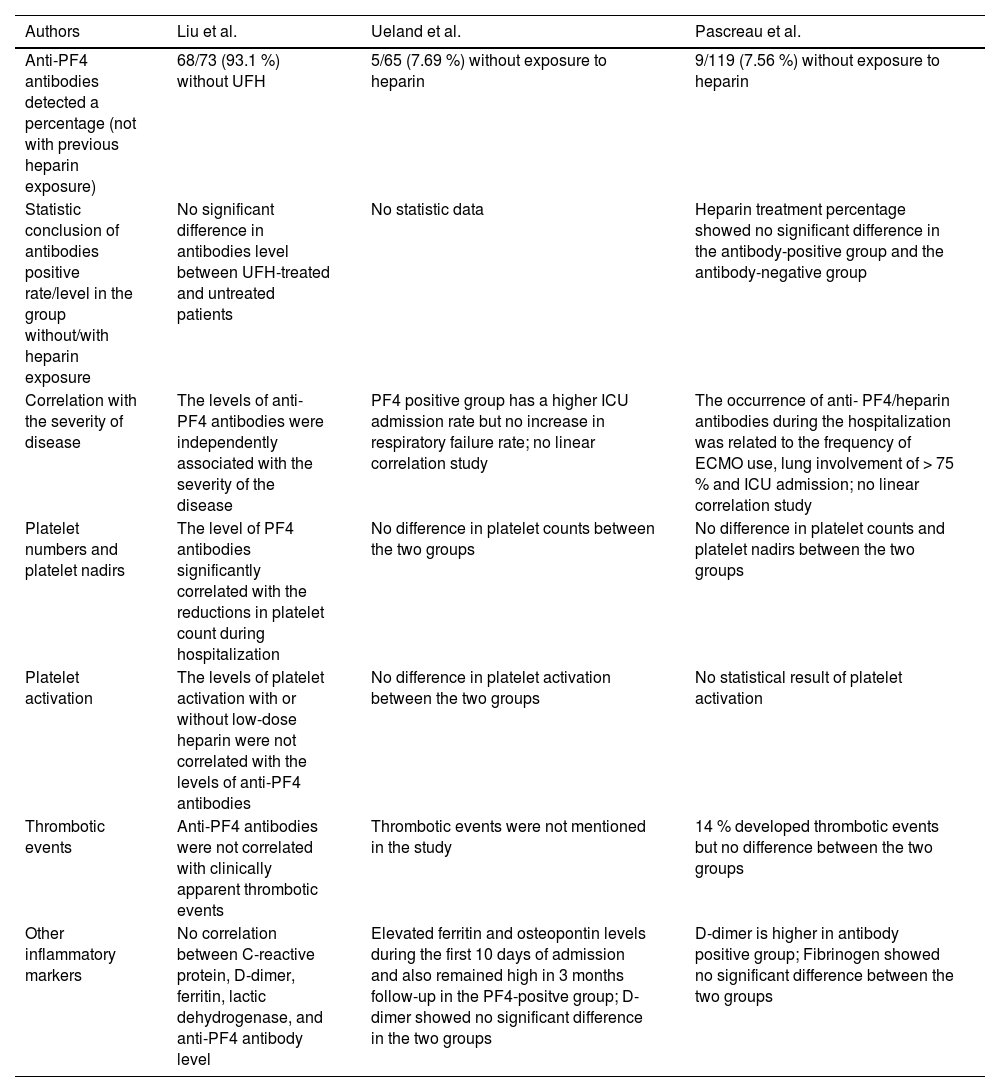
In the Cohort study of Liu et al., anti-PF4 antibodies were detected in 95 % of the patients included, and 93.1 % showed no previous exposure to ultrahigh frequency (UFH).44 Compared to the prevalence rate of 93.1 %, the other two articles from Ueland et al. and Pascreau et al. reported lower prevalence rates in patients with COVID-19 without heparin exposure, which are 7.69 % and 7.56 %, respectively.45,46 These three articles also have similar conclusions in many aspects. One of the conclusions is that anti-PF4 antibodies are related to the disease severity. According to the above articles, different measurements are used for the disease severity. In Liu's article, the levels of anti-PF4 antibodies were independently associated with the severity of the disease.44 On the other hand, both Ueland and Pascreau's studies showed significant differences in the ICU admission.45,46 The second consensus is the difference in thrombotic events, as all three articles also concluded that anti-PF4 antibodies are unrelated to thrombotic events.44-46 Interestingly, inflammatory markers and other laboratory tests in these three articles do not arrive at the same conclusion regarding the correlation with anti-PF4 antibodies, including platelet counts, platelet nadir, D-dimer and other inflammatory markers. Only Liu's cohort illustrated a significant platelet number decrease in the group with anti-PF4 antibodies.44 In contrast, only Pascreau's study reported a higher D-dimer in the antibody-positive group.46 Elevation of ferritin and osteopontin levels was found in Ueland's study.45 We summarized the above discussion in Table 2. However, the reasons behind the varying prevalence of anti-PF4 antibodies in patients with COVID-19 are still unknown. We can hypothesize that anti-PF4 antibodies can be present in patients diagnosed with COVID-19. They are related to the severity of the disease, but neither to the HIT nor to thrombotic events.
Results of the three studies related to PF4 antibodies and COVID-19 (unrelated to HIT).
In Table 1, there are two other articles related to this topic, but the selection of patients in these two articles in patients suspected of HIT, which means exposure to heparin, was present when data were collected.47,48 Among ten patients tested by Nazy et al. in their study, the IgG-specific immune complex-mediated reaction causing platelet activation was confirmed by the serotonin release assay (SRA). The platelet activation was not induced by heparin, but was inhibited by both therapeutic and high-dose heparin, which was discussed as a possible reason behind the critical illness of COVID-19. However, in their study, only three samples showed weakly positive IgG-specific anti-PF4/heparin antibodies, compared to six critically ill COVID-19 samples that demonstrated platelet activation.47 It is still unclear if anti-PF4 antibodies play a role in platelet activation. A large cohort in 2023 showed anti-PF4 antibody levels were within the normal range in the convalescent plasma from post-COVID-19-infected patients.49 However, the plasma collections were from patients after 28 days post-COVID-19 infection, without thrombotic complications, and fully recovered, which could not reflect the acute phase of the COVID-19 infection.49
ConclusionThe traditional concept of HIT is now transforming into a broad concept as an anti-PF4 disorder and includes a broad spectrum of diagnoses. Recently, we have had more studies focusing on VITT and its mechanism, but only some discussed the direct relationship between the COVID-19 infection and anti-PF4 antibodies. In our article, we summarized the articles related to this novel topic. Even without heparin exposure, some patients with COVID-19 infection have still created anti-PF4 antibodies and some evidence shows it is related to the severity of the disease. However, the anti-PF4 antibodies in these patients are unrelated to thrombotic events or platelet activation. Mechanisms and theories behind this phenomenon can be targeted in future studies.
All authors contributed to the drafting of the manuscript, approved of its final version, and had final responsibility for the decision to submit for publication. We thank Dr. Yun Yen who provided special help for organizing and reviewing the manuscript.