Approximately 55.52% of the Indian population had been fully vaccinated by Jan. 2022, since its first roll out on January 16, 2021. A few concerns were raised concerning the Covishield vaccination related to thrombotic thrombocytopenia. Apheresis-derived platelet concentrates are frequently required in a plethora of clinical situations and post-vaccination decrement of platelet counts might lead to increased deferral of the platelet-pheresis donors. Objectives. The aim of the study was to discover the effect of the Covishield vaccination on deferral rates of plateletpheresis donors.
MethodsBlood samples were collected from the potential platelet donors for the completion of the standard questionnaire for the complete blood count. The data collected were tabulated in the MS Excel spreadsheet and the biostatistical analysis was performed with the SPSS v23. A p-value of < 0.05 was taken as significant. We compared this data with age- and sex-matched controls.
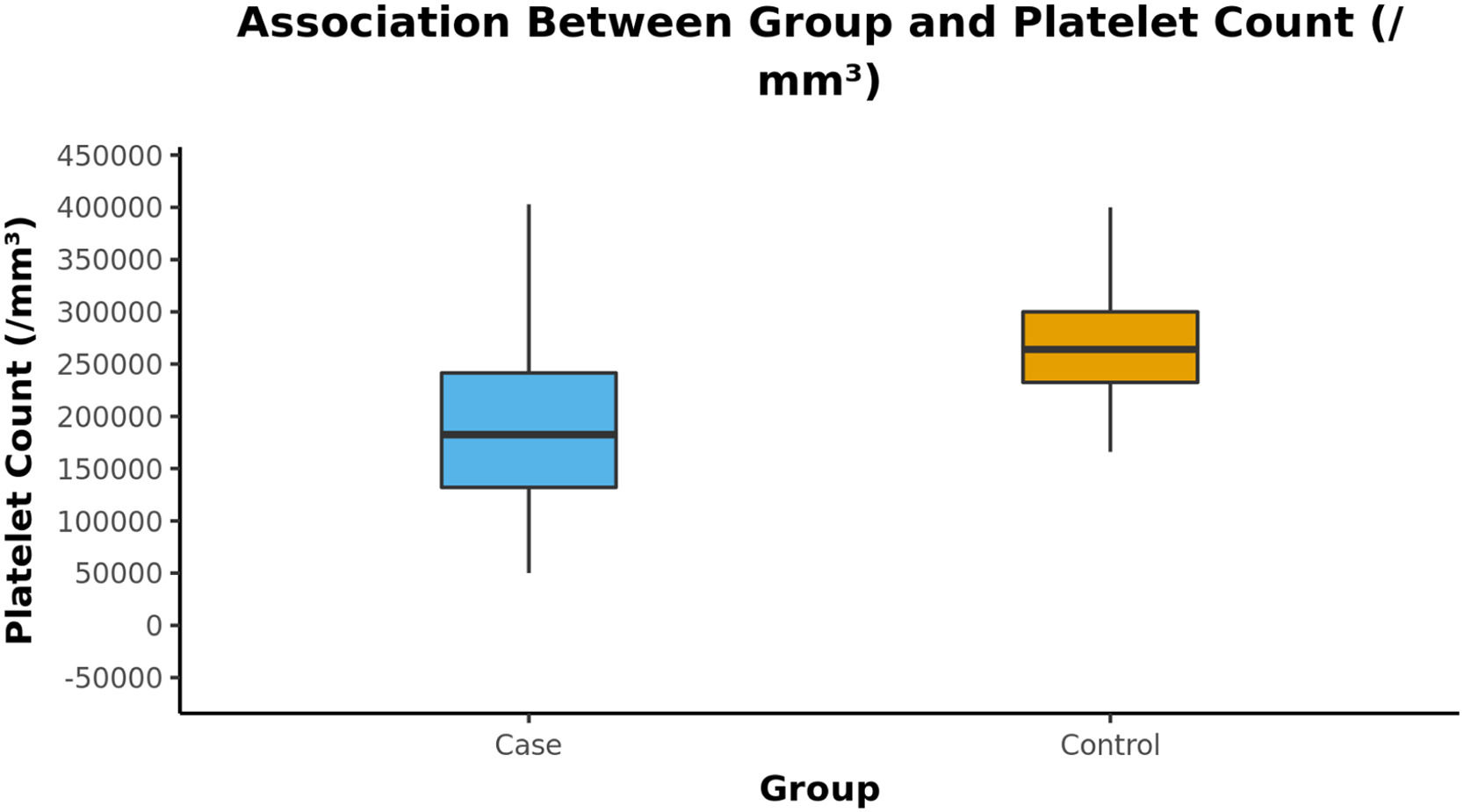
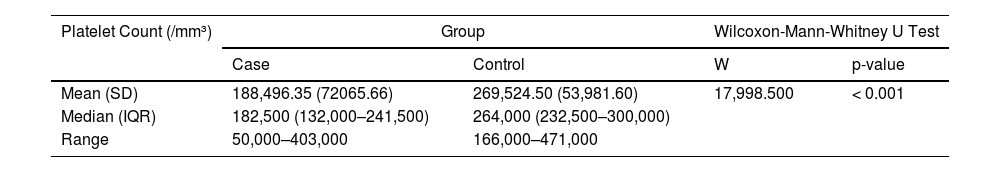
ResultsThe mean age of cases and controls was 29.69 ± 8.57 and 30.15 ± 7.11, respectively. There was a significant difference in platelet counts of cases (188496.35 ± 72065.66/cumm) and controls (269524.50 ± 53981.60/cumm). Furthermore, donors who received one dose had higher platelet counts of 248676.47 ± 80075.24/cumm than those who received both doses of vaccine (179970.83 ± 66773.73/cumm) . The difference in deferral rates between the two groups was remarkable (34.7% vs. 0.9%, with the p-value < 0.001).
ConclusionVaccination certainly increased the deferral rates of plateletpheresis donors due to low platelet counts. Average platelet counts were low in fully vaccinated individuals, however, the platelets returned to normal counts as the post-vaccination days progressed.
The ongoing COVID-19 pandemic provided the impetus to drive one of the biggest and most extensive global vaccine development programs in history, leading to the development of various vaccines against the Coronavirus.1,2 A widespread vaccination program commenced in India, predominantly at the Serum Institute of India (SII), which manufactured ‘Covishield’ (the name employed in India for the Oxford-AstraZeneca vaccine), on Jan. 16, 2021, with the banner of the free and largest vaccination drive globally.3,4
An interim analysis of four randomized controlled trials in Brazil, South Africa and the UK showed the safety, immunogenicity and efficacy data of ChAdOx1, administered in two doses containing 5 × 1010 viral particles to 23,745 participants aged 18 years or older in clinical studies outside India, which showed the vaccine efficacy to be 70.42%.5
Most of the adverse reactions observed after vaccination, such as headache, dizziness, fever, myalgia, flu-like symptoms and tenderness at the injection site, were transient in nature and lasted for less than 72 hours in most cases.6 The actual issue started when Europe and nearby countries reported multiple cases of vaccine-induced thrombocytopenia (VIT) and unusual thrombotic episodes (cerebral venous sinus and splanchnic vein).7,8 Researchers found close linkage to immune-mediated thrombocytopenia as a causative factor of low platelet counts and pathogenesis, akin to heparin-induced thrombotic thrombocytopenia (HITT), as many patients demonstrated the presence of antibodies against PF4 (similar to HITT). They were managed with intravenous immunoglobulin, steroids and non-heparin anticoagulants, with no platelet transfusion. Even though there were a few reported fatalities, the majority of the cases recovered without any sequelae.9
There are 7 reported cases of vaccine-induced thrombotic thrombocytopenia in India and further individual case reports are emerging with raised D-Dimer and PF4 IgG antibody levels.10-12 However, no research publication revealed the impact of only thrombocytopenia on plateletpheresis donors. We undertook this case-control study to demonstrate the effect of the Covishield vaccination on the deferral rates of our healthy voluntary platelet donors.
MethodsOur center is one of the largest government-owned transfusion centers, with 3 dependent multispecialty hospitals, for which we serve as a mother blood center, including a cardiothoracic and bone marrow transplant facility, when requirements of apheresis-derived platelets are indispensable. We are completely dependent upon voluntary platelet donors to meet this huge demand for platelet concentrate units.
In this study, we took participants among willing platelet donors vaccinated in 2021, after fulfilling the eligibility criteria, as per the National Blood Transfusion Council 2017 revised donor selection guidelines. The comparison group was our non-vaccinated age and sex-matched controls from the year 2019 (from the available plateletpheresis donor database), as there was no COVID-19 or vaccination against COVID-19 during that period. This subgroup was chosen as a control because platelet donations during 2020 at our center were very low due to the fear of COVID-19 spread and cancellation of planned surgeries. The comparison group might have been much smaller (< 100), as compared to the sample size (274). In addition to this, barring COVID-19 and vaccination, all other eligibility criteria were matched between the two groups.
After obtaining informed consent from our donors, all the cases were provided with a questionnaire to acquire details about their demographic data, vaccination, travel and COVID-19 histories, ongoing medications (if any), especially those which affect the platelet number or function, any bleeding or anticoagulant intake histories and recent platelet counts (if they recollected). Only after scrutinizing these questionnaires and ensuring that none of the donors had coexisting medical conditions or were on medications that could impact their platelet counts, were they advised to provide this for the complete blood count (CBC) before platelet donation to check the donor hematocrit and platelet counts. The control group data was already available in our database (donor questionnaire forms and platelet donation register) for the year 2019, such as ongoing medications, medical history, negative history of bleeding tendencies, family history of bleeding disorders, etc. (except COVID-19-related questions).
After deferring donors who had coexisting bleeding tendencies or any of the ongoing COVID-19 symptoms based on the questionnaire, we selected 274 cases for the period between Feb. 2021 to Aug. 2021 and for the same time period in 2019, amounting to 374 age- and sex-matched controls, which we used for comparison for this study. The matching was achieved using donor questionnaire forms for both cases and controls manually by the one-to-one method. The cut-off for platelet donor selection at our center is a platelet count of > 1.5 lacs/mm³.
The cases and control data were tabulated in an MS Excel spreadsheet with the IBM SPSS statistics software v23 for the evaluation, using statistical tools to obtain the deferral rate and correlation between vaccination and thrombocytopenia. We obtained ethical clearance from the Institutional Ethical Committee. Chi-squared tests were used to make group comparisons in normally distributed data tables, whereas nonparametric tests, such as the Wilcoxon-Mann-Whitney U and Kruskal-Wallis tests were used in data that is not normally distributed to discover the p-value. A p-value < 0.05 was considered significant. Associations and correlations were depicted using the Spearman Correlation Coefficient with the Rho and p-value to establish the strength of correlation.
ResultsThere was not a major difference between the age of participants and controls, as we had matched them to avoid the confounding factor as can be seen in Table 1.
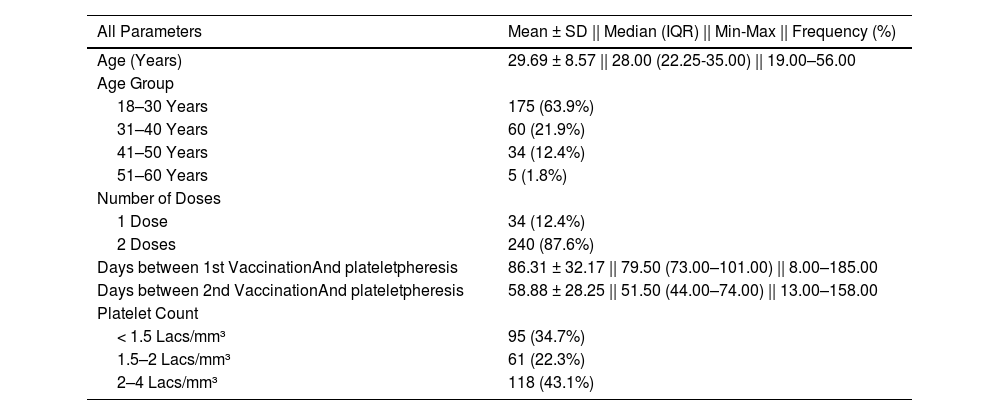
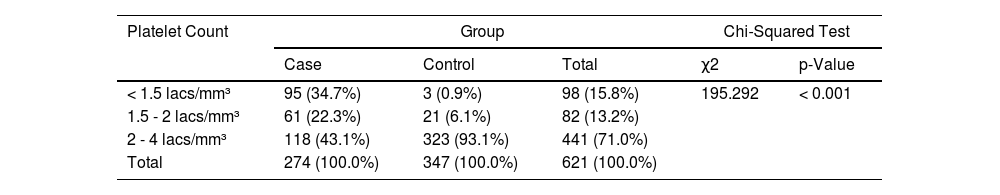
The descriptive statistics of the cases (vaccinated group) are presented in Table 2. The mean days after the 1st and 2nd doses of vaccination and plateletpheresis were 86.31 ± 32.17 and 58.88 ± 28.25, respectively. A total of 95 (34.7%) of the participants had platelet counts < 1.5 Lacs/mm³ (which led to their deferral).
. Descriptive statistics of the cases (vaccinated group).
| All Parameters | Mean ± SD || Median (IQR) || Min-Max || Frequency (%) |
|---|---|
| Age (Years) | 29.69 ± 8.57 || 28.00 (22.25-35.00) || 19.00–56.00 |
| Age Group | |
| 18–30 Years | 175 (63.9%) |
| 31–40 Years | 60 (21.9%) |
| 41–50 Years | 34 (12.4%) |
| 51–60 Years | 5 (1.8%) |
| Number of Doses | |
| 1 Dose | 34 (12.4%) |
| 2 Doses | 240 (87.6%) |
| Days between 1st VaccinationAnd plateletpheresis | 86.31 ± 32.17 || 79.50 (73.00–101.00) || 8.00–185.00 |
| Days between 2nd VaccinationAnd plateletpheresis | 58.88 ± 28.25 || 51.50 (44.00–74.00) || 13.00–158.00 |
| Platelet Count | |
| < 1.5 Lacs/mm³ | 95 (34.7%) |
| 1.5–2 Lacs/mm³ | 61 (22.3%) |
| 2–4 Lacs/mm³ | 118 (43.1%) |
The mean (SD) of Platelet Count (/mm³) in the cases was 188,496.35 (72,065.66). The mean (SD) of Platelet Count (/mm³) in the control group was 269,524.50 (53,981.60). There was a significant difference between the two groups in terms of platelet count (/mm³) (W = 17,998.500, p = < (perguntar para autor Shouldn't this be “≤” ??? 0.001) (Table 3).
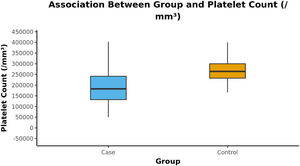
The Box-and-Whisker plot below depicts the distribution of the platelet counts (/mm³) in the 2 groups. The upper and lower extent of the whiskers represent the Tukey limits for the platelet counts (/mm³) in each of the groups (Figure 1).
We applied the chi-square test to discover the association between cases and controls and the platelet counts to see the effect of vaccination. There was a significant difference between the two groups in terms of distribution of platelet counts (χ2 = 195.292, p = < 0.001) (Shouldn't this be “≤” ??? (Table 4).
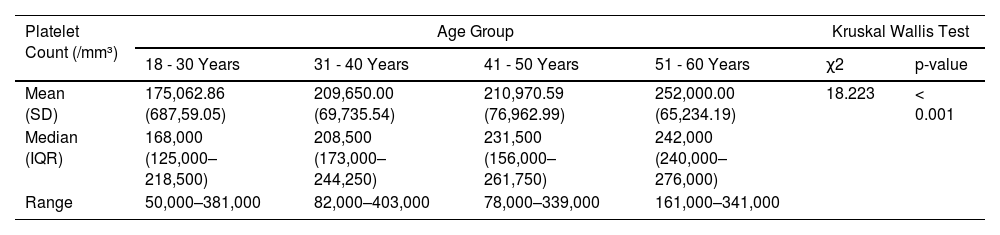
We also compared the age groups of donors with platelet counts and found that young donors in the vaccinated group had lower counts, as compared to donors aged > 50 yrs. The variable platelet count (/mm³) was not normally distributed in the 4 subgroups, as shown in Table 5.
Comparison of the age groups in the cases, as to their platelet counts (Kruskal Wallis Test).
| Platelet Count (/mm³) | Age Group | Kruskal Wallis Test | ||||
|---|---|---|---|---|---|---|
| 18 - 30 Years | 31 - 40 Years | 41 - 50 Years | 51 - 60 Years | χ2 | p-value | |
| Mean (SD) | 175,062.86 (687,59.05) | 209,650.00 (69,735.54) | 210,970.59 (76,962.99) | 252,000.00 (65,234.19) | 18.223 | < 0.001 |
| Median (IQR) | 168,000 (125,000–218,500) | 208,500 (173,000–244,250) | 231,500 (156,000–261,750) | 242,000 (240,000–276,000) | ||
| Range | 50,000–381,000 | 82,000–403,000 | 78,000–339,000 | 161,000–341,000 | ||
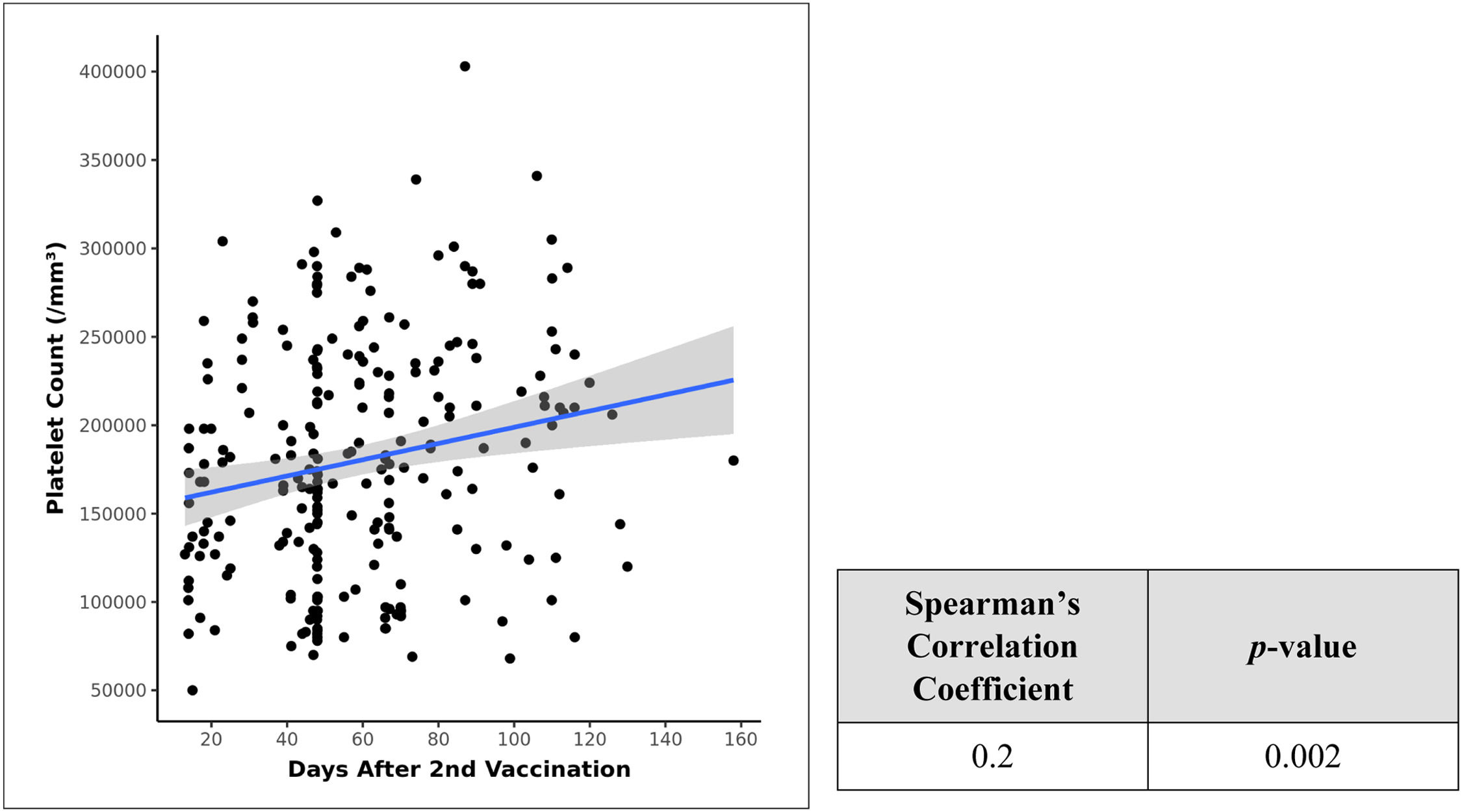
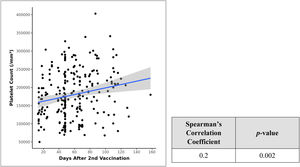
There was a marked difference between the platelet counts after the 1st and 2nd doses of vaccination, as can be seen in the Table 2 descriptive statistics. Therefore, we tried to correlate the days after vaccination with resultant platelet counts and applied the Spearman's correlation coefficient in a scatter plot and found a weak positive correlation between days after the 2nd dose of vaccination and platelet counts (lacs/mm³) and this correlation was statistically significant (rho = 0.2, p = 0.002) (Figure 2). No similar significant statistical relationship exists between days after the 1st dose and platelet counts.
DiscussionReports related to AstraZeneca ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant), also known as Covishield (manufactured and marketed by Serum Institute of India), causing thrombotic thrombocytopenia, appeared in the initial months of 2021.13
As per the official press release of AstraZeneca, cases of thromboembolic events have been reported following the administration of ChAdOx1 nCoV-19 vaccine in several EEA countries, some leading to local suspensions of specific batches or to the use of the vaccine itself.7 Although the pathophysiology is not yet clear, data from Greinacher et al. suggested that some constituents in the ChAdOx1 nCov-19 vaccine can form antigenic complexes with platelet factor 4 (PF4) (proven from biophysical analysis) and immune complexes were recognized by antibodies from the thrombotic thrombocytopenia syndrome patients.13,14 This has raised concerns regarding the safety of the vaccine.
In our study, we found that the vaccine under reference indeed led to decreased platelet counts and had caused deferral of almost in 35% of the eligible platelet donors, when compared to the non-vaccinated control group. We kept cases and controls in the same demographic profile to see if this effect was due to vaccination or not, avoiding all known confounding variables to the best of our knowledge, using one-to-one matching of donor questionnaire forms. Since both our groups are matched for all other variables affecting platelet counts, the probability of thrombocytopenia due to vaccination deserves merit. This deferral of the donor population increased the paucity of platelet donation during this pandemic, which led to a crisis-like situation for our center. Being a government center, our donor pool was large and we somehow managed the platelet stock inventory by carefully selecting the donors with adequate platelet counts, but we assumed this problem might have affected the already meagre platelet supply across the country, especially the apheresis-derived platelet concentrates.15
Our study is the only study that actually compared the platelet counts post-vaccination and analyzed the effect on the deferral rate. Hence, no comparison data is available on PUBMED, Google Scholar, DOAJ, PLOS one or Science direct. This study also revealed that the 2nd dose of vaccination affected the platelet counts more than the first dose and, with each passing day, the counts improved and reached beyond the threshold limit of donation after 90 days. The age-related difference in platelet counts post-vaccination is an interesting finding which suggested that young donors are affected more than donors past their 5th decade of life. The reason for this is not clear, but might be due to some immunological mechanisms that entails younger individuals to be more immunocompetent, giving rise to more anti-PF4 antibodies, as compared to the older individuals, and better immunity, which in turn leads to stronger immune response and lower platelet counts.
ConclusionOur study suggested that vaccine-induced thrombocytopenia is real and can lead to increased deferral of voluntary platelet donors, although we cannot comment upon the thrombotic profile, as we have not followed our donors post-donation or deferral. The study needs to be performed at a multicentric level, so as to derive the conclusion regarding the association between vaccine and thrombocytopenia at a national level.