The COVID-19 pandemic had an important impact on blood bank services. The onset of the pandemic led to a decrease in the number of blood donors. A remote interview would avoid deferred donors from having to travel to the blood bank. We evaluate the feasibility of using telemedicine as an alternative to a face-to-face interview as a first blood donor screening.
MethodsOur retrospective study included 404 whole blood and platelets donors, who underwent the clinical interview remotely via telemedicine. The deferred donor would not need to go to the blood bank and eligible candidates were required to donate within 7 days. On the day of donation, a mini-interview was held to ensure donor and blood safety.
ResultsThe appointments were made from June 2020 to June 2022, including 263 candidates for whole blood (WB) and 141 for platelets (PLTs). At the end of the telemedicine interview, 285 (70.6 %) candidates were considered eligible. Telemedicine was not performed for 60 (14.8 %) candidates due to technical problems (with audio or video) or absences. The deferral rate among candidates who underwent telemedicine pre-screening was 14.6 % and, among eligible donors after telemedicine, only 7 (2.9 %) were unable to donate blood.
ConclusionTelemedicine is a viable alternative and a welcome convenience for potential donors to avoid unnecessary travel.
A fundamental activity for blood banks is the monitoring of the blood supply. This was a major concern during the coronavirus disease 2019 (COVID-19) pandemic. One study supports that patients with COVID-19 do not require additional transfusions, compared to non-COVID-19 patients.1 In our service, we observed a reduction in the transfusion demand associated with a drop in patient care activities. Thus, the 30 % drop in the number of blood donations was not problematic. Over the months, maintaining an adequate supply of blood components became critical when the increase in the total number of transfused units did not also occur in the number of blood donations.
In Brazil, we have public and private blood banks. This service may be located within a hospital or at a blood center that will distribute blood to affiliated hospitals. An interview for blood donors is mandatory by law and donation must be voluntary, altruistic and unpaid. The prospective donor is required to meet the qualification requirements. We measure blood pressure, pulse and temperature and collect a drop of blood to test the hemoglobin level to ensure the donor is eligible to donate blood. The donors are also required to answer confidential questions about their medical history to ensure that the donation is safe for them and that the blood is safe for transfusion to others. A blood sample from each allogeneic donation is tested for anti-HBc, HBsAg, HBV NAT (nucleic acid testing), anti-HCV, HCV NAT, anti-HIV1/2, HIV NAT, anti-HTLV-I/II, T. cruzi antibodies and syphilis (non-treponemal or treponemal assays).
During the COVID-19 pandemic, donors were afraid of contracting the virus, despite all the hygiene and safety measures adopted by the hospitals. To donate blood, it is mandatory to undergo a prior screening, which is performed in person. Therefore, potential donors are required to go to blood services. Some services outside Brazil already use alternative approaches to the in-person questionnaire, with computerized systems, in which the donors perform their own screening.2 There are also mixed systems, in which some questions are answered in the computer system and others, on more sensitive topics, such as risky sexual behavior, in the face-to-face mode.2 In all situations, the donor must personally respond to the health screening.
Telemedicine is a remote delivery of healthcare services via the telecommunications infrastructure and has grown exponentially during the pandemic caused by the new coronavirus SARS-CoV-2. In the United States, the number of telehealth consultations increased by 50 % in the first quarter of 2020 and by 154 % in week 13 in 2020, compared to the same period of 2019.3 In Brazil, until then, telemedicine was governed by regulations restricting its use. In 2020, new ordinances and regulations were sanctioned on an emergency basis, increasing its scope of action.
A pre-screening interview was proposed for potential blood donors using telemedicine, which is a remote interview, to assess whether the donor was able to donate blood before going to the blood bank. Based on our 2019 historical data, our service had an ineligibility rate among potential donors of 20 %. Among the deferrals, 80 % were possible to identify solely by interview, the other 20 % were due to low or high Hb, high blood pressure, etc., situations that only a face-to-face assessment would detect. With this implementation, we would avoid deferred donors going to the blood bank after the interview. This would minimize the risk of exposure, protecting healthcare workers and blood donors and reducing the visits of ineligible donors to the blood bank.
According to the World Health Organization (WHO), Brazil is the third country in number of cases and the second in number of deaths from COVID-19.4 In this scenario, new approaches were implemented to mitigate the impact of COVID-19 in several health sectors. The pre-screening interview of blood donors by telemedicine is not a practice of blood banks, however, it was a strategy adopted by our blood bank to face this global situation. The objective of this study was to show the service's experience with telemedicine and to assess whether it is a viable and safe alternative.
Material and methodsStudy designA retrospective study was conducted at the Hospital Israelita Albert Einstein in Brazil. We collected data from all telemedicine pre-screening scheduled from June 2020 to June 2022.
Data collectionWe collected data from donors who scheduled telemedicine, such as sex, age, type of donation (whole blood (WB) or platelets (PLTs)), status (eligible, deferral, technical problems during telemedicine or not performed), if he/she were a first-time donor (a donor who had never donated blood before) and if he/she were a first-time donor at our service (a donor who had donated blood before, but never at our service).
We also collected data on the day of donation from candidates who were eligible after pre-screening on whether the donation was performed, systemic adverse events, result of infectious disease markers and confidential unit exclusion (CUE). The CUE is a suggestion by the Brazilian Ministry of Health and is an opportunity for the donor confidentially to self-exclude for reasons of increased risk not reported or deliberately omitted during screening. The donor is asked if he/she is comfortable having his/her blood transfused into a recipient – two options: “Yes” or “No”. If the answer is “No”, we collect the blood and perform tests, but, during the processing, the bag is discarded. If the answer is “Yes”, the blood will be transfused.
Telemedicine interviewAfter the onset of the COVID-19 pandemic, we offered to blood donors the opportunity to undergo the clinical interview remotely. They scheduled an appointment on our website or over the phone and then received instructions by e-mail. If he/she were a first-time donor at our service, a pre-registration was required. The candidates were instructed on the need to remain in a quiet and reserved place during the interview. Telemedicine was performed by the blood bank healthcare workers, also in a private room of the blood bank. Deferred candidates would not need to go to the blood bank and the eligible candidates were required to donate within seven days.
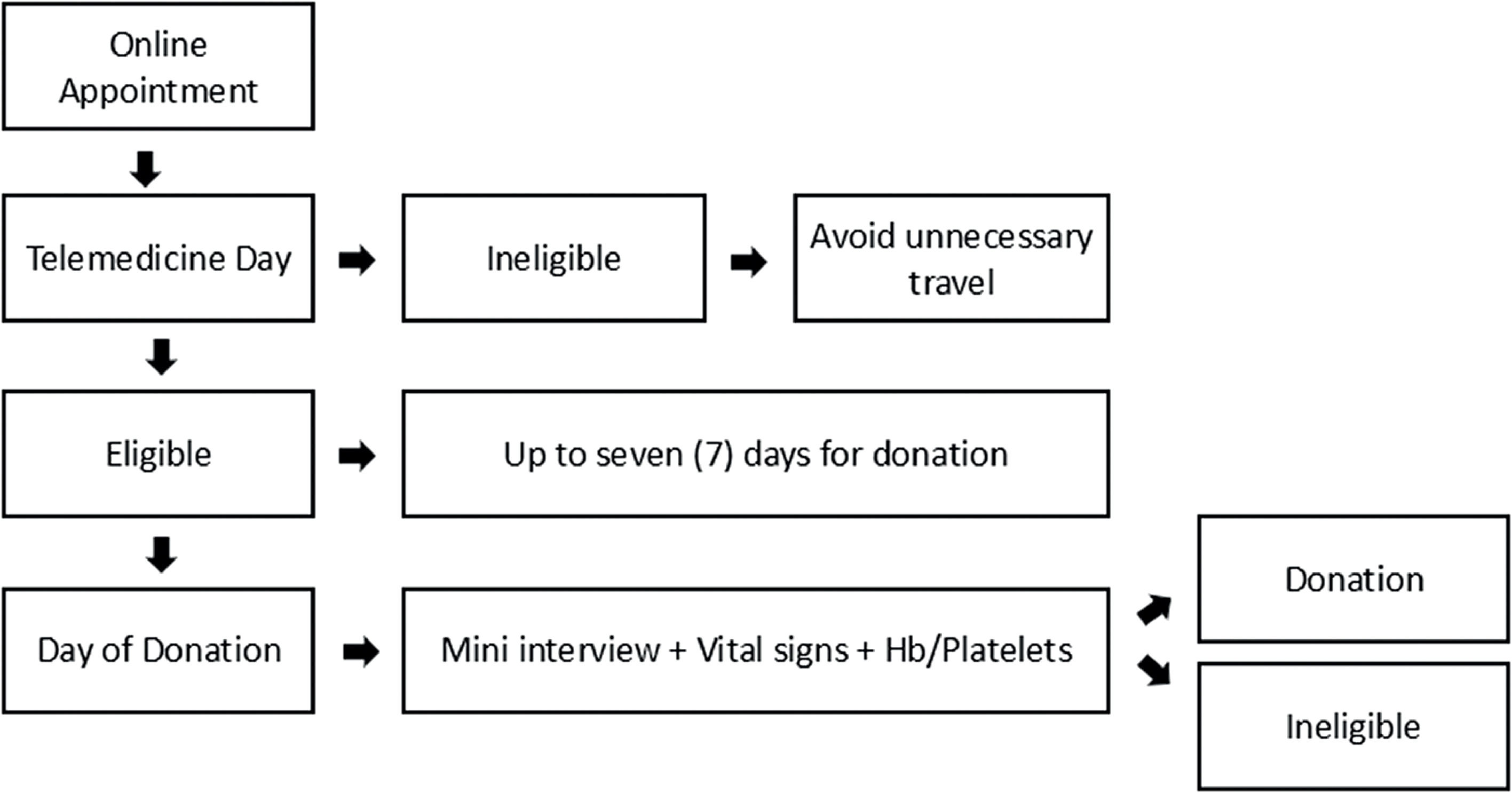
Day of donationAt the blood bank, we confirmed the interval between telemedicine and donation, personal information, verified the identity document and took a photo (for the first-time donors). If this interval had exceeded seven days, the candidate was required to undergo the entire clinical interview again. Otherwise, the candidate would perform a mini- interview with eleven questions to ensure the safety of the donor and the donated blood. Vital signs (pulse, blood pressure and temperature) were assessed and the hemoglobin level (for WB and PLT donation) and platelet count (for PLT donation) were determined to confirm eligibility for the donation. Subsequently, donors answered the confidential unit exclusion question. Informed consent was required prior to the blood collection. The flowchart is presented in Figure 1.
Ethical considerationThe study was approved by the Research Ethics Committee of the Hospital Israelita Albert Einstein in the Certificado de Apresentação de Apreciação Ética (CAAE) no. 53642921.0.0000.0071.
Statistical analysisAll these data were available in our computerized blood bank system. The demographic data of the donors were automatically extracted by the e-Progesa (Mak-System) by a blood bank employee. The researcher received the data on an Excel spreadsheet. The donors were coded and anonymized. The data analysis was performed in blocks, with the researcher guaranteeing the confidentiality of the data on the research participants.
The evaluated characteristics were described for all volunteers using absolute and relative frequencies, only age was described using the mean ± standard deviation. Contingency tables were created by crossing information of interest using absolute and relative frequencies. Demographic characteristics were described according to years and the association with the use of chi-square tests was verified. Ages were compared using the Student's t-test. Analyses were performed using the SPSS software version 22.0 and tests were performed with a significance level of 5 %.
ResultsA total of 404 appointments were made from June 2020 to June 2022, with 263 candidates for WB and 141 for PLTs. At the end of the telemedicine interview, 285 candidates were considered eligible and 59 had donation deferrals. In this last group, 53 were candidates for WB and 6, for platelets. Telemedicine was not employed for 14.8 % of the candidates due to technical problems (with audio or video with 13 donors) or absences (47 donors who had scheduled telemedicine appointments and did not attend them, but also had not canceled).
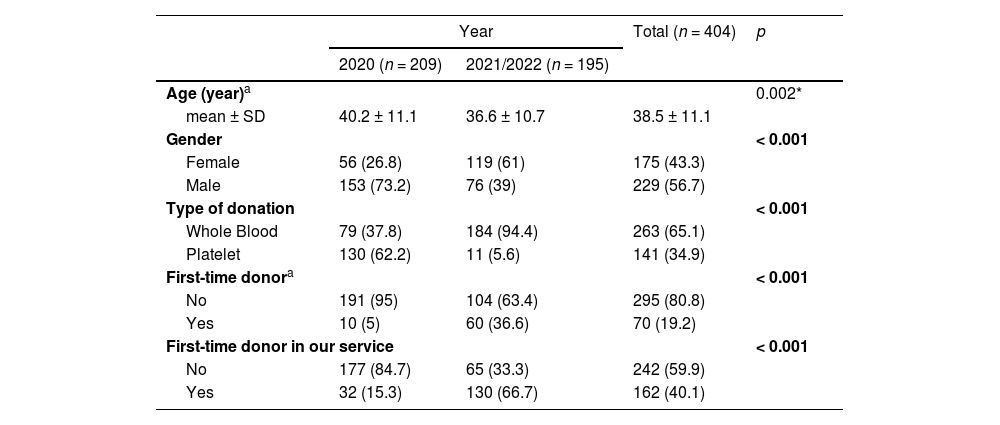
During the seven-month period of 2020, the most prevalent donors who underwent telemedicine pre-screening were platelet donors and male donors, 62.2 % and 73.2 %, respectively. In 2021 and in the five months of 2022, 94.4 % of the donors wanted to donate WB and 61 % were female. Furthermore, in 2021/2022 66.7 % of the candidates were first-time donors at our service, in contrast with 15.3 % in 2020 (Table 1).
Characteristics of telemedicine donors.
| Year | Total (n = 404) | p | ||
|---|---|---|---|---|
| 2020 (n = 209) | 2021/2022 (n = 195) | |||
| Age (year)a | 0.002* | |||
| mean ± SD | 40.2 ± 11.1 | 36.6 ± 10.7 | 38.5 ± 11.1 | |
| Gender | < 0.001 | |||
| Female | 56 (26.8) | 119 (61) | 175 (43.3) | |
| Male | 153 (73.2) | 76 (39) | 229 (56.7) | |
| Type of donation | < 0.001 | |||
| Whole Blood | 79 (37.8) | 184 (94.4) | 263 (65.1) | |
| Platelet | 130 (62.2) | 11 (5.6) | 141 (34.9) | |
| First-time donora | < 0.001 | |||
| No | 191 (95) | 104 (63.4) | 295 (80.8) | |
| Yes | 10 (5) | 60 (36.6) | 70 (19.2) | |
| First-time donor in our service | < 0.001 | |||
| No | 177 (84.7) | 65 (33.3) | 242 (59.9) | |
| Yes | 32 (15.3) | 130 (66.7) | 162 (40.1) | |
Chi-square test.
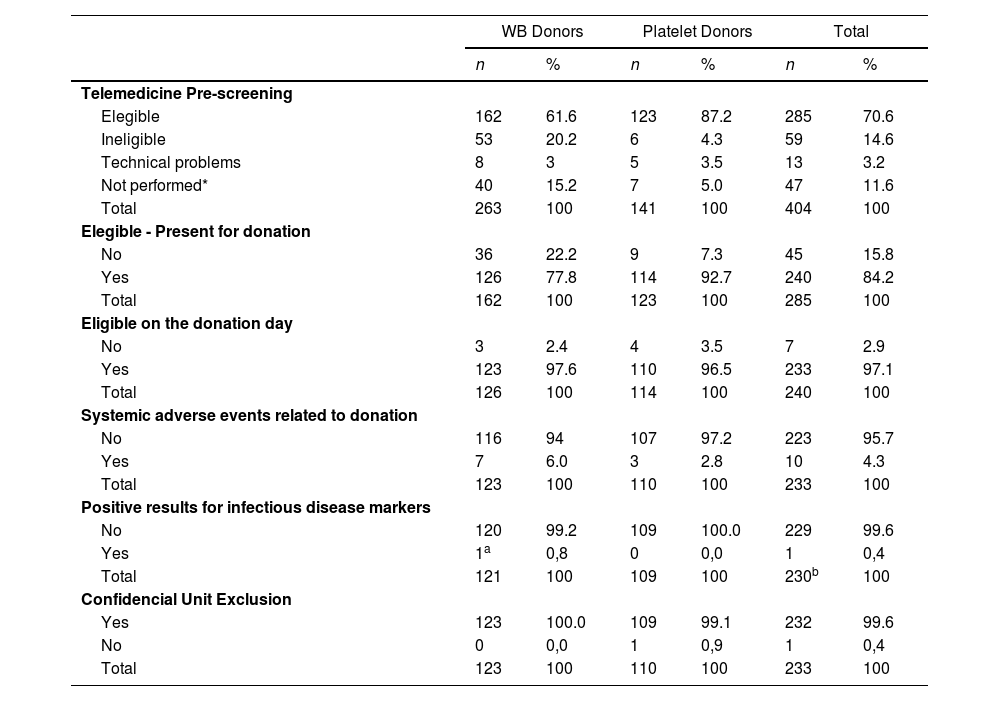
Among the 285 eligible candidates after the telemedicine evaluation, 240 attended the blood bank and 233 were eligible for donation. Four donors could not donate PLTs, as two had platelet counts < 150,000mm3, one had an Hb count of <12.5mg/dL and one had a skin lesion. Among the WB donors, two donors were deferred for presenting Hb counts of <12.5mg/dL and one donor, for weighing <50kg. The ineligibility rate among candidates who was eligible after telemedicine was only 2.9 %. The no-show rate of eligible donors was 15.8 % (Table 2).
Results.
| WB Donors | Platelet Donors | Total | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Telemedicine Pre-screening | ||||||
| Elegible | 162 | 61.6 | 123 | 87.2 | 285 | 70.6 |
| Ineligible | 53 | 20.2 | 6 | 4.3 | 59 | 14.6 |
| Technical problems | 8 | 3 | 5 | 3.5 | 13 | 3.2 |
| Not performed* | 40 | 15.2 | 7 | 5.0 | 47 | 11.6 |
| Total | 263 | 100 | 141 | 100 | 404 | 100 |
| Elegible - Present for donation | ||||||
| No | 36 | 22.2 | 9 | 7.3 | 45 | 15.8 |
| Yes | 126 | 77.8 | 114 | 92.7 | 240 | 84.2 |
| Total | 162 | 100 | 123 | 100 | 285 | 100 |
| Eligible on the donation day | ||||||
| No | 3 | 2.4 | 4 | 3.5 | 7 | 2.9 |
| Yes | 123 | 97.6 | 110 | 96.5 | 233 | 97.1 |
| Total | 126 | 100 | 114 | 100 | 240 | 100 |
| Systemic adverse events related to donation | ||||||
| No | 116 | 94 | 107 | 97.2 | 223 | 95.7 |
| Yes | 7 | 6.0 | 3 | 2.8 | 10 | 4.3 |
| Total | 123 | 100 | 110 | 100 | 233 | 100 |
| Positive results for infectious disease markers | ||||||
| No | 120 | 99.2 | 109 | 100.0 | 229 | 99.6 |
| Yes | 1a | 0,8 | 0 | 0,0 | 1 | 0,4 |
| Total | 121 | 100 | 109 | 100 | 230b | 100 |
| Confidencial Unit Exclusion | ||||||
| Yes | 123 | 100.0 | 109 | 99.1 | 232 | 99.6 |
| No | 0 | 0,0 | 1 | 0,9 | 1 | 0,4 |
| Total | 123 | 100 | 110 | 100 | 233 | 100 |
Regarding donor safety, we had 10 systemic adverse events, all being mild, with no moderate or severe ones. Among the WB donors, whose mean age was 32 years old, there were five mild vasovagal reactions, three of which occurred in first-time donors. Among the PLT donors, one donor presented mild lipothymia and two, paresthesia, one of whom required oral calcium. On blood safety, we had only one positive result for infectious disease markers. It was a positive Anti-HBc. After confirmatory tests, we concluded that it was a false positive test. Three donors had incomplete donations, so we did not perform tests for them. A PLT donor with three previous donations answered “No” to the confidential unit exclusion (CUE) (Table 2).
DiscussionOur study showed that telemedicine for blood donors is a viable and efficient alternative. The deferral rate among candidates who were eligible after telemedicine pre-screening was only 2.9 %. Our deferral rate after the face-to-face interview in 2020 was 20 %. The conduction of the telemedicine interview does not exhaust the reasons for ineligibility, since it is still possible to present deferral due to changes in the hemoglobin level/platelet count, vital signs or the physical examination. Therefore, it proved to be effective in identifying candidates with clinical ineligibility. And it is safe for the donor, with no moderate or severe systemic adverse events related to the donation, and for the recipient, with no true positive results for infectious disease markers.
We find two different donor scenarios in 2020 and 2021/2022. At the beginning of the pandemic during seven months of 2020, we contacted our donors, mainly PLT donors, and offered this new strategy, so we had more male donors (73.2 % in 2020 and 39 % in 2021/022, p < 0.001) and no first-time donors (5 % in 2020 and 36.6 % in 2021/2022, p < 0.001). After a few months, during 2021 and 2022, it became a strategy for all donors. Telemedicine primarily attracted younger (36.6 ± 10.7 years old in 2021/2022 and 40.2 ± 11.1 years old in 2020, p = 0.002), WB (37.8 % in 2020 and 94.4 % in 2021/2022, p < 0.001) and first-time donors, who actively sought our homepage. In 2021/2022, 66.7 % of the candidates who adhered to telemedicine had never donated blood at our service before.
Sümning et al. reported that social media had become the second most important motivator for recruiting first-time donors.5 In the beginning, this remote interview was a strategy to convince blood donors to continue donating and that it was safe to go to the blood bank. Over time, we realized that this had also become a recruitment strategy for new donors. A systematic review on blood donation incentives found that attitudes towards incentives are mixed and depend on the donor`s age and experience.6 Telemedicine can be an incentive to facilitate the blood donation process and a strategy to attract younger donors.
We did not use sophisticated equipment, just a computer with a webcam and headphones, but it was necessary to make some adjustments. We needed to hire an online appointment site and a telemedicine system. It was also necessary to create new questions and to conduct a mini-screening (on the day of the donation after the telemedicine screening), which also involved the training of the healthcare workers. After the first few months, the process became easy and practical.
This study had some limitations. We had a limited number of participants. We promoted this strategy only on our homepage, so we did not reach a large audience, but with this endeavor we realized a new opportunity to attract and possibly retain young donors. We aim to expand telemedicine in the future. Some donors were unable to undergo the telemedicine evaluation due to technological barriers, difficulty in navigating online or in installing the system on cell phones, in addition to slow connections resulting in poor video and audio quality and loss of connection. Furthermore, nearly 16 % of the eligible donors did not present themselves for donation within seven days, this represents an opportunity to improve candidate awareness of blood donation.
ConclusionsThe aim of this study was to show our experience with the remote pre-screening interview of potential blood donor. Telemedicine can be a viable and safe alternative and a welcome convenience for potential donors to avoid unnecessary travel, in addition to being a possible strategy to recruit new and younger donors.