To verify the association between the ABO blood type and the risk of SARS-CoV-2 infection and COVID-19 disease severity.
MethodsThis review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), using the 2020 PRISMA Checklist and flow diagram, and articles selected for review were analyzed using the Newcastle-Ottawa Quality Rating Scale. The research question was: “Would the ABO blood group influence the risk of infection and clinical course of patients infected with SARS-CoV-2?”, The following databases were used: Embase, PubMed, Virtual Health Library (VHL), Web of Science, ScienceDirect and Scopus. The protocol for this review was registered in the Prospective Register of Systematic Reviews (PROSPERO), number CRD42021245945.
ResultsWe found 798 articles across PubMed, Embase, Scopus, Web of Science, Science Direct and Virtual Health Library and 54 articles were included in the final analysis. Among 30 studies evaluating the risk of COVID-19 infection, 21 found significant correlations with ABO blood groups, 14 of them revealing an increased risk in blood group A and 15 studies showing a decreased risk in blood group O. Most studies found no significant correlation with disease severity or mortality.
ConclusionThe qualitative assessment of available information suggests that blood group A may be a risk factor for COVID-19 infection and that blood group O may have a protective effect. We were unable to determine a clear association between the ABO blood group and mortality. These conclusions are based on highly heterogenous evidence.
Coronavirus Disease 19 (COVID-19) is an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which may present with fever, cough and dyspnea or asymptomatic infection in some cases. The SARS-CoV-2 belongs to the Coronaviridae family, being an enveloped RNA virus, which contains Spike (S) proteins on its surface that make it look like a crown (Corona). It has high homology to the coronavirus responsible for the SARS outbreak of 2003, Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1).1
Although most cases present with mild symptoms, the COVID-19 infection may lead to acute respiratory failure and death. This disease has caused worldwide health and economic impact, leading the international community to unite in trying to contain the virus through restrictive measures, research in potential drugs and mass vaccination. Despite these efforts, there is still a large number of new cases of the disease. Thus, it is important to continue searching for risk factors that might be associated with worse outcomes in persons infected by SARS-CoV-2.1
Individuals with comorbidities and the elderly are susceptible to severe COVID-19 infection, but one of the intriguing aspects of this disease is that healthy young people may sometimes present severe respiratory failure, while many people with comorbidities may be asymptomatic.2
One of the hypotheses to explain susceptibility to SARS-CoV-2 is linked to the ABO blood group system, represented by ABH oligosaccharide antigens A and B and antibodies against these antigens (anti-A and anti-B). These antigens are coded by autosomal codominant genes corresponding to alleles A and B in the ABO locus. The H antigen, coded by FUT1/FUT2/SEC1 genes, is responsible for exhibiting A and B antigens with a α−1,3-glycosidic bond. When the A allele is present, one N-acetyl-d-galactosamine (GalNAc) or, when the B allele is present, a d-galactose (Gal) is transferred to the H antigen.1 These antigens are mainly expressed on the surface of red blood cells, on the surface of endothelial and epithelial cells and in mucins secreted by exocrine glandules.
The main objectives of this systematic review were to investigate the association between the ABO blood group and the risk of COVID-19 infection and the association between the ABO blood group and the risk of suffering complications and death related to COVID-19.
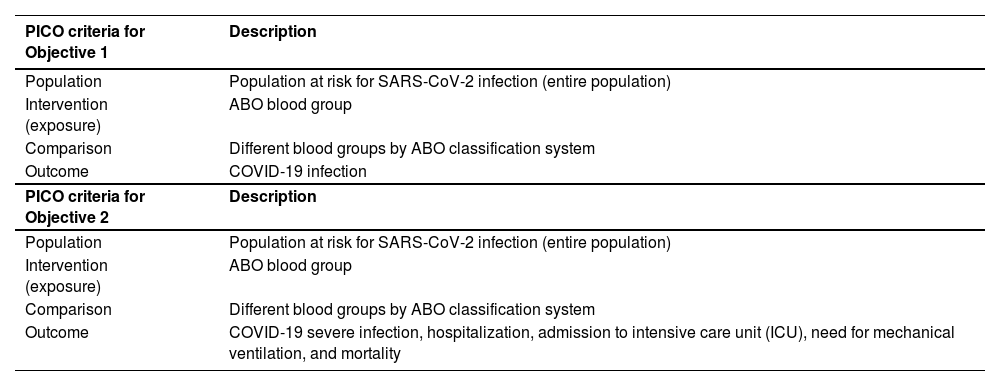
Materials and methodsThis study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines through the 2020 PRISMA Checklist and 2020 PRISMA Flow diagram. The protocol for this review was registered in the Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42021245945. The research question was structured and refined using the PICO method (Population, Intervention/exposure, Comparison and Outcome), presented as follows: “Would the ABO blood group influence the risk of infection and clinical course of patients infected with SARS-CoV-2?”, as detailed in Tables 1 and 2.
PICO strategy used to construct the research.
Search strategy of the articles used in this research.
PICO strategy for objectives 1 and 2 are as follows:
PICO strategy for objective 1 (COVID-19 infection): P: Population at risk for SARS-CoV-2 infection (entire population); I: ABO blood group; C: Different blood groups by ABO classification system, and; O: COVID-19 infection.
PICO strategy for objective 2 (COVID-19 severe infection): P: Population at risk for SARS-CoV-2 infection (entire population); I: ABO blood group; C: Different blood groups by ABO classification system, and; O: COVID-19 severe infection, hospitalization, admission to intensive care unit (ICU), need of mechanical ventilation, cardiovascular injury and mortality.
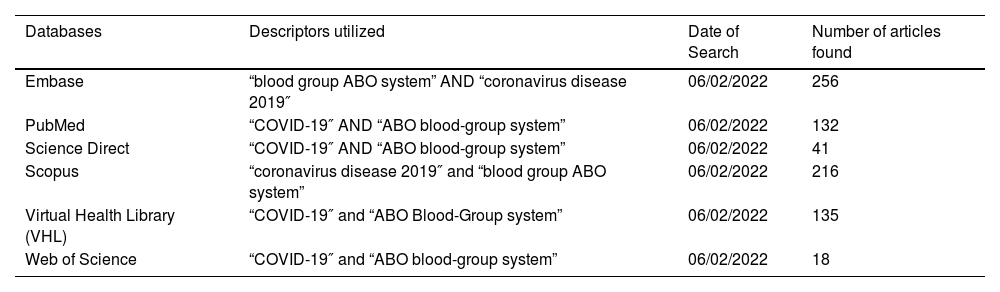
The following databases were used: Embase, PubMed, Virtual Health Library (VHL), Web of Science, ScienceDirect and Scopus. The search strategy was based on paired combinations of the following descriptors, using the Boolean operator “AND”: “COVID-19″, “Coronavirus disease 2019″, “ABO blood-group system” and “blood group ABO system”. Articles in English, Spanish and Portuguese were included. The search was limited to articles published from January 2020 to December 2021. Details on the article collection strategy are in Table 3.
Evaluation of included studies by Newcastle-Ottawa Scale (NOS).
| Authors (year) | Type of study | Selection | Comparability | Outcome |
|---|---|---|---|---|
| RAY et al. (2020)14 | Cohort | **** | ** | *** |
| ZIETZ et al. (2020)15 | Cohort | *** | ** | *** |
| SARDU et al. (2020)13 | Cohort | ** | ** | *** |
| BARNKOB et al. (2020)16 | Cohort | *** | * | *** |
| MUÑIZ-DIAZ et al. (2020)5 | Cohort | **** | ** | *** |
| TEVATIA et al. (2020)17 | Cohort | **** | * | ** |
| NAUFFAL et al. (2021)18 | Cohort | **** | ** | *** |
| NALBANT et al. (2022)19 | Cohort | **** | ** | *** |
| AMOROSO et al. (2021)20 | Cohort | **** | * | *** |
| SZYMANSKI et al. (2021)21 | Cohort | *** | * | *** |
| BADEDI et al. (2021)22 | Cohort | *** | * | *** |
| DE FREITAS DUTRA et al. (2021)23 | Cohort | ** | * | ** |
| DOMÈNECH-MONTOLIU et al. (2021)24 | Cohort | ** | * | *** |
| TAMAYO-VELASCO et al. (2021)25 | Cohort | ** | ** | ** |
| ISHAQ et al. (2021)26 | Cohort | ** | * | *** |
| KABRAH et al. (2021)59 | Cohort | *** | * | *** |
| MATZHOLD et al. (2021)27 | Cohort | *** | * | ** |
| MULLINS et al. (2021)28 | Cohort | ** | * | ** |
| GRECO et al. (2021)29 | Cohort | **** | ** | ** |
| AL‐YOUHA et al. (2020)30 | Cohort | *** | ** | ** |
| FAN et al. (2020) (31) | Case control | **** | ** | *** |
| AD'HIAH et al. (2020)32 | Case control | **** | ** | *** |
| ZHAO et al. (2020) (33) | Case control | **** | * | *** |
| SAIFY et al. (2020)34 | Case control | **** | ** | * |
| WU et al. (2020)35 | Case control | ** | * | *** |
| KHALIL et al. (2020)36 | Case control | ** | ** | ** |
| BADEDI et al. (2020)37 | Case control | *** | ** | *** |
| BEHBOUDI et al. (2021)38 | Case control | ** | ** | * |
| TORRES- ALARCÓN et al. (2021)39 | Case control | ** | * | ** |
| KİRİŞCİ et al. (2021)40 | Case control | ** | ** | ** |
| KOLIN et al. (2020)41 | Case control | **** | ** | ** |
| GAMBOA-AGUILAR et al. (2022)42 | Case control | *** | *** | ** |
| LATZ et al. (2020)43 | Cross-sectional | ** | ** | ** |
| LIU et al. (2020)6 | Cross-sectional | ** | * | ** |
| HOILAND et al. (2020)44 | Cross-sectional | *** | ** | ** |
| GÖKER et al. (2020)45 | Cross-sectional | *** | ** | ** |
| KOTILA et al. (2020)46 | Cross-sectional | ** | * | ** |
| YAYLACI et al. (2020)47 | Cross-sectional | * | ** | ** |
| SOLMAZ et al. (2020)48 | Cross-sectional | *** | * | ** |
| AL KUWARI et al. (2020)49 | Cross-sectional | *** | ** | ** |
| ALMADHI et al. (2021)50 | Cross-sectional | *** | ** | ** |
| AKTIMUR et al. (2020)51 | Cross-sectional | *** | ** | ** |
| ESREF et al. (2020)52 | Cross-sectional | *** | * | ** |
| MUÑOZ-CULLA et al. (2021)53 | Cross-sectional | *** | * | ** |
| KHASAYESI et al. (2021)54 | Cross-sectional | ** | * | ** |
| PASANGHA et al. (2020)55 | Cross-sectional | * | ** | ** |
| KOMAL et al. (2021)56 | Cross-sectional | ** | * | * |
| HALIM et al. (2021)57 | Cross-sectional | *** | * | ** |
| HERMEL et al. (2021)58 | Cross-sectional | *** | ** | ** |
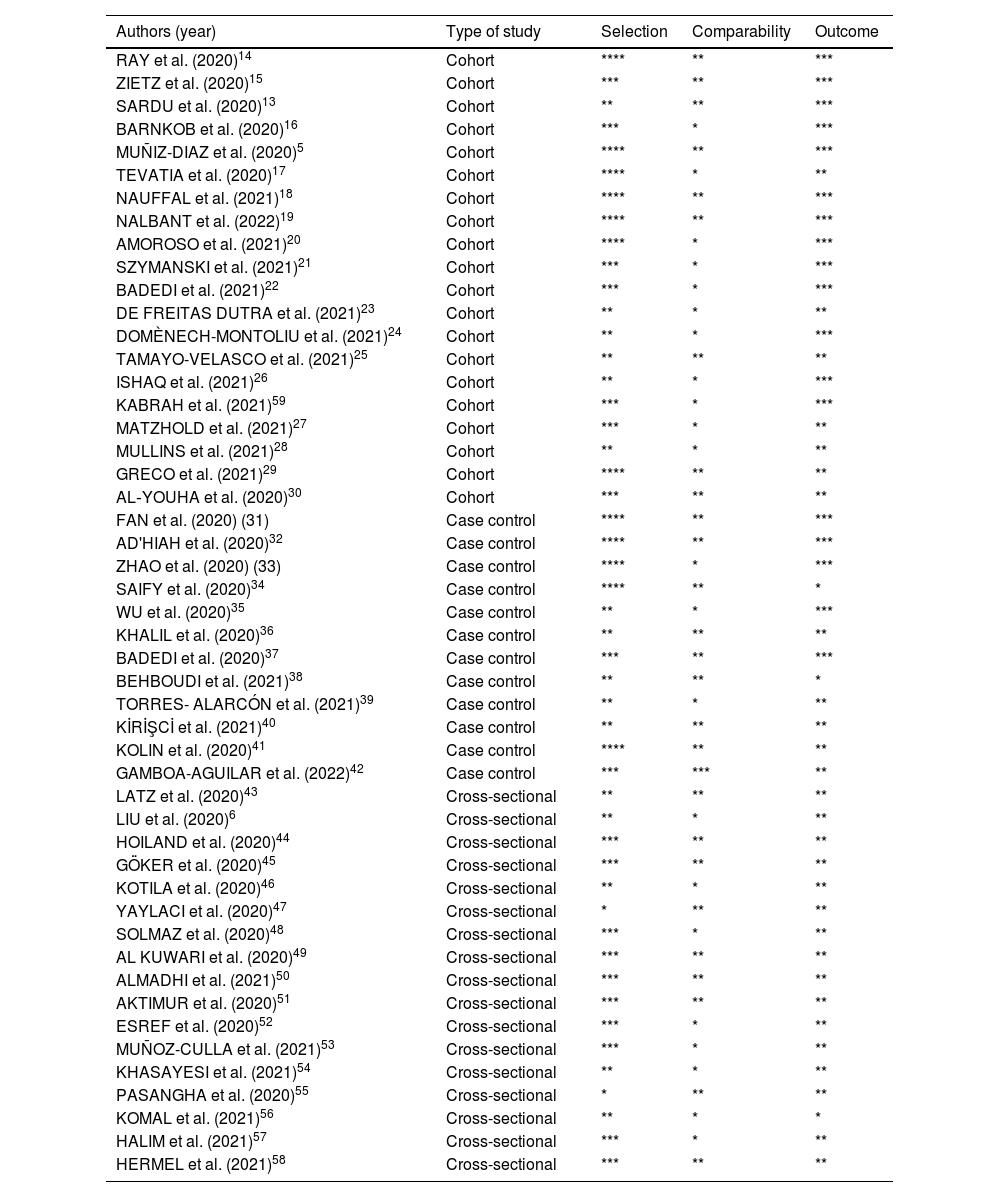
Note: Quality assessment using the NOS scale for Cohort and Case Control Studies revealed that all included studies scored from 6 (ranging from 0 to 9 points) and for Cross-sectional Studies, using the adapted NOS, all scored from 5 (ranging from 0 to 7 points).7 Therefore, all studies had good scientific quality.
The following outcome was considered for the first objective (risk of COVID-19 infection):
- •
COVID-19 infection determined by either polymerase chain reaction (PCR), antigen or antibody tests.
For the second objective (COVID-19 severity) the following outcomes were considered:
- •
Mortality
- •
Admission to intensive care unit (ICU)
- •
Need for mechanical ventilation
We included studies with humans of any age and of both sexes who manifested symptoms of SARS-CoV-2 infection, confirmed by laboratory tests, and that have reported ABO blood group types. Case–control, cohort and cross-sectional studies were eligible for inclusion. Studies were required to provide data for at least one pre-established outcome variable in order to be eligible for inclusion.
We then excluded review articles, short communications, commentaries and editorial and opinion papers. Published studies with coronavirus infected animals, treatment with convalescent plasma, women in a gestational state (due to physiological changes typical of that period) and symptomatic patients without laboratory confirmation were also excluded.
Strategy for data extractionFirst, three reviewers (DMBS, JLBS and RBM) performed a literature search using the descriptors mentioned above. The reviewers then removed the duplicates and all remaining titles and abstracts were evaluated for eligibility. If this was insufficient to determine the eligibility, full-text articles were retrieved. The full texts of the articles that met the inclusion criteria were analyzed separately by six reviewers (DMBS, DABSA, EMBS, FCAN, MSNP and VCVG). Any disagreement among them about the eligibility of certain studies was resolved through discussion with two other reviewers (GFA and PRN).
Risk quality assessment and bias reportThe methodological quality of the studies was assessed by two reviewers and, in the case of a disagreement, another reviewer was asked for further analysis. Data provided for the systematic review were verified using the Newcastle-Ottawa Quality Rating Scale3 by previously trained and qualified reviewers. The methodological quality scores of the cross-sectional, cohort and case-control studies were calculated in three components: selection of groups (0 - 4 points), quality of fit for confounding factors (0 - 2 points) and assessment of exposure after outcome (0 - 3 points). The maximum score was 7 points for cross-sectional studies and 9 points for cohort and case-control studies,3 which represents high methodological quality. The results of this analysis are contained in Table 4.
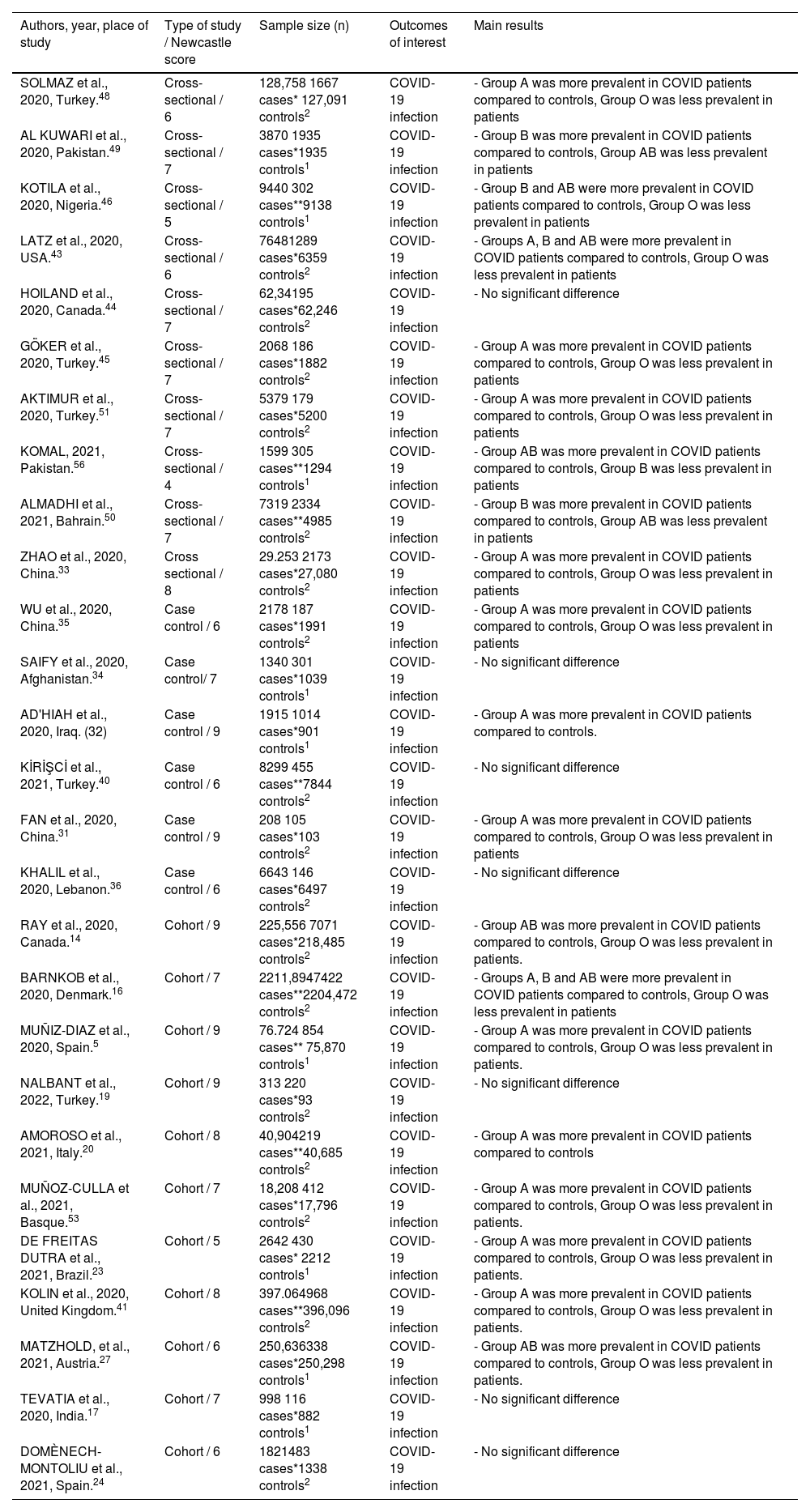
Studies that show correlation between ABO blood group and COVID infection.
| Authors, year, place of study | Type of study / Newcastle score | Sample size (n) | Outcomes of interest | Main results |
|---|---|---|---|---|
| SOLMAZ et al., 2020, Turkey.48 | Cross-sectional / 6 | 128,758 1667 cases* 127,091 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| AL KUWARI et al., 2020, Pakistan.49 | Cross-sectional / 7 | 3870 1935 cases*1935 controls1 | COVID-19 infection | - Group B was more prevalent in COVID patients compared to controls, Group AB was less prevalent in patients |
| KOTILA et al., 2020, Nigeria.46 | Cross-sectional / 5 | 9440 302 cases**9138 controls1 | COVID-19 infection | - Group B and AB were more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| LATZ et al., 2020, USA.43 | Cross-sectional / 6 | 76481289 cases*6359 controls2 | COVID-19 infection | - Groups A, B and AB were more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| HOILAND et al., 2020, Canada.44 | Cross-sectional / 7 | 62,34195 cases*62,246 controls2 | COVID-19 infection | - No significant difference |
| GÖKER et al., 2020, Turkey.45 | Cross-sectional / 7 | 2068 186 cases*1882 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| AKTIMUR et al., 2020, Turkey.51 | Cross-sectional / 7 | 5379 179 cases*5200 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| KOMAL, 2021, Pakistan.56 | Cross-sectional / 4 | 1599 305 cases**1294 controls1 | COVID-19 infection | - Group AB was more prevalent in COVID patients compared to controls, Group B was less prevalent in patients |
| ALMADHI et al., 2021, Bahrain.50 | Cross-sectional / 7 | 7319 2334 cases**4985 controls2 | COVID-19 infection | - Group B was more prevalent in COVID patients compared to controls, Group AB was less prevalent in patients |
| ZHAO et al., 2020, China.33 | Cross sectional / 8 | 29.253 2173 cases*27,080 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| WU et al., 2020, China.35 | Case control / 6 | 2178 187 cases*1991 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| SAIFY et al., 2020, Afghanistan.34 | Case control/ 7 | 1340 301 cases*1039 controls1 | COVID-19 infection | - No significant difference |
| AD'HIAH et al., 2020, Iraq. (32) | Case control / 9 | 1915 1014 cases*901 controls1 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls. |
| KİRİŞCİ et al., 2021, Turkey.40 | Case control / 6 | 8299 455 cases**7844 controls2 | COVID-19 infection | - No significant difference |
| FAN et al., 2020, China.31 | Case control / 9 | 208 105 cases*103 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| KHALIL et al., 2020, Lebanon.36 | Case control / 6 | 6643 146 cases*6497 controls2 | COVID-19 infection | - No significant difference |
| RAY et al., 2020, Canada.14 | Cohort / 9 | 225,556 7071 cases*218,485 controls2 | COVID-19 infection | - Group AB was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| BARNKOB et al., 2020, Denmark.16 | Cohort / 7 | 2211,8947422 cases**2204,472 controls2 | COVID-19 infection | - Groups A, B and AB were more prevalent in COVID patients compared to controls, Group O was less prevalent in patients |
| MUÑIZ-DIAZ et al., 2020, Spain.5 | Cohort / 9 | 76.724 854 cases** 75,870 controls1 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| NALBANT et al., 2022, Turkey.19 | Cohort / 9 | 313 220 cases*93 controls2 | COVID-19 infection | - No significant difference |
| AMOROSO et al., 2021, Italy.20 | Cohort / 8 | 40,904219 cases**40,685 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls |
| MUÑOZ-CULLA et al., 2021, Basque.53 | Cohort / 7 | 18,208 412 cases*17,796 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| DE FREITAS DUTRA et al., 2021, Brazil.23 | Cohort / 5 | 2642 430 cases* 2212 controls1 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| KOLIN et al., 2020, United Kingdom.41 | Cohort / 8 | 397.064968 cases**396,096 controls2 | COVID-19 infection | - Group A was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| MATZHOLD, et al., 2021, Austria.27 | Cohort / 6 | 250,636338 cases*250,298 controls1 | COVID-19 infection | - Group AB was more prevalent in COVID patients compared to controls, Group O was less prevalent in patients. |
| TEVATIA et al., 2020, India.17 | Cohort / 7 | 998 116 cases*882 controls1 | COVID-19 infection | - No significant difference |
| DOMÈNECH-MONTOLIU et al., 2021, Spain.24 | Cohort / 6 | 1821483 cases*1338 controls2 | COVID-19 infection | - No significant difference |
Outcome variables were expressed as the proportion of all selected patients with at least one event. We did not perform a meta-analysis due to the high heterogeneity of data.
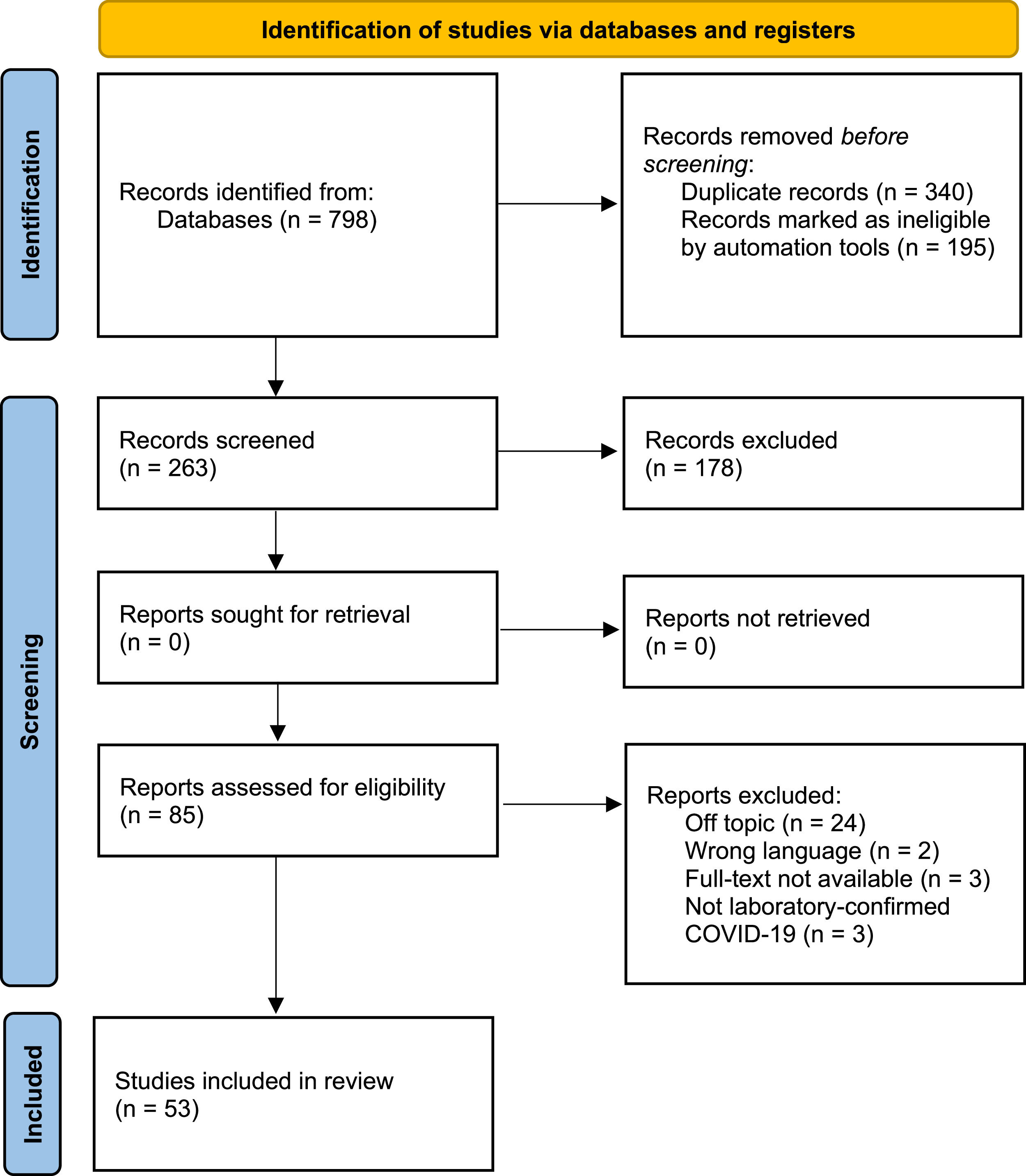
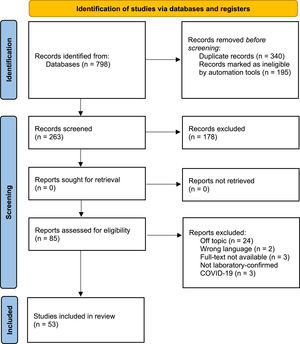
ResultsThe initial search resulted in 798 articles across the following databases: PubMed, Embase, Scopus, Web of Science, Science Direct and Virtual Health Library. Then automation tools were applied (detailed in Figure 1), resulting in the exclusion of 195 articles. The next step was duplicate removal, which further excluded 340 articles. Subsequently, 263 studies were selected for title and abstract reading and 178 of those were excluded for lacking pertinence to the subject of our research, not fulfilling inclusion criteria or for matching any of the exclusion criteria, resulting in 85 manuscripts for full-text reading. Fifty-three matched the research question of this review and were included in the final analysis. Figure 1 details the search and selection flow-chart. Three study types were analyzed: Cohort studies (n = 22), Cross-sectional studies (n = 20) and case-control studies (n = 11).
For objective 1 (risk of COVID-19 infection) 30 articles were analyzed (Table 4). Sample sizes ranged from 208 to 2211,894 participants, totaling 2212,102 participants. These studies were conducted in 19 countries and the majority were from Turkey (6 studies), China (3 studies) and Spain (3 studies). The mean age ranged from 5.8 years (5 months - 13.3 years) to 82 years (6.9 years).4,5 Regarding the type of studies found, 11 were cohort, 12, cross-sectional, and 7, case control. Different types of samples were found. Some involved hospitalized or discharged patients, while others evaluated outpatients.
Among 30 studies evaluating the risk of COVID infection associated with the ABO blood type, 21 studies found significant correlations. Most studies (14 studies), involving 105 to 7422 patients, found an increased proportion of blood group A in patients, compared to controls. Fewer studies showed an increased proportion of patients with blood group B (6 studies) or AB (6 studies). Most studies also found a reduced proportion of blood group O (15 studies, 105 – 7422 patients) in infected patients. A total of 10 studies found no statistically significant correlation between ABO status and COVID-19 infection.
Among studies with blood donors as controls (10 studies), most studies found a reduced proportion of patients with blood group O (4 studies, 302 - 854 patients). Fewer studies showed an increased proportion of patients with blood group A (3 studies), B (2 studies) or AB (2 studies). Two out of 10 studies found no statistically significant correlation between the ABO status and COVID-19 infection using blood donors as controls.
The majority of the studies (10 studies with 105 – 7422 patients) with non-blood donor controls (21 studies) found an increased proportion of blood group A in patients, compared to controls. Fewer studies showed an increased proportion of patients with blood group B (3 studies) or AB (3 studies). Most studies also found a reduced proportion of patients with blood group O (11 studies, 105 – 7422 patients). Seven studies using non-blood donors as controls found no statistically significant correlation between the ABO status and COVID infection.
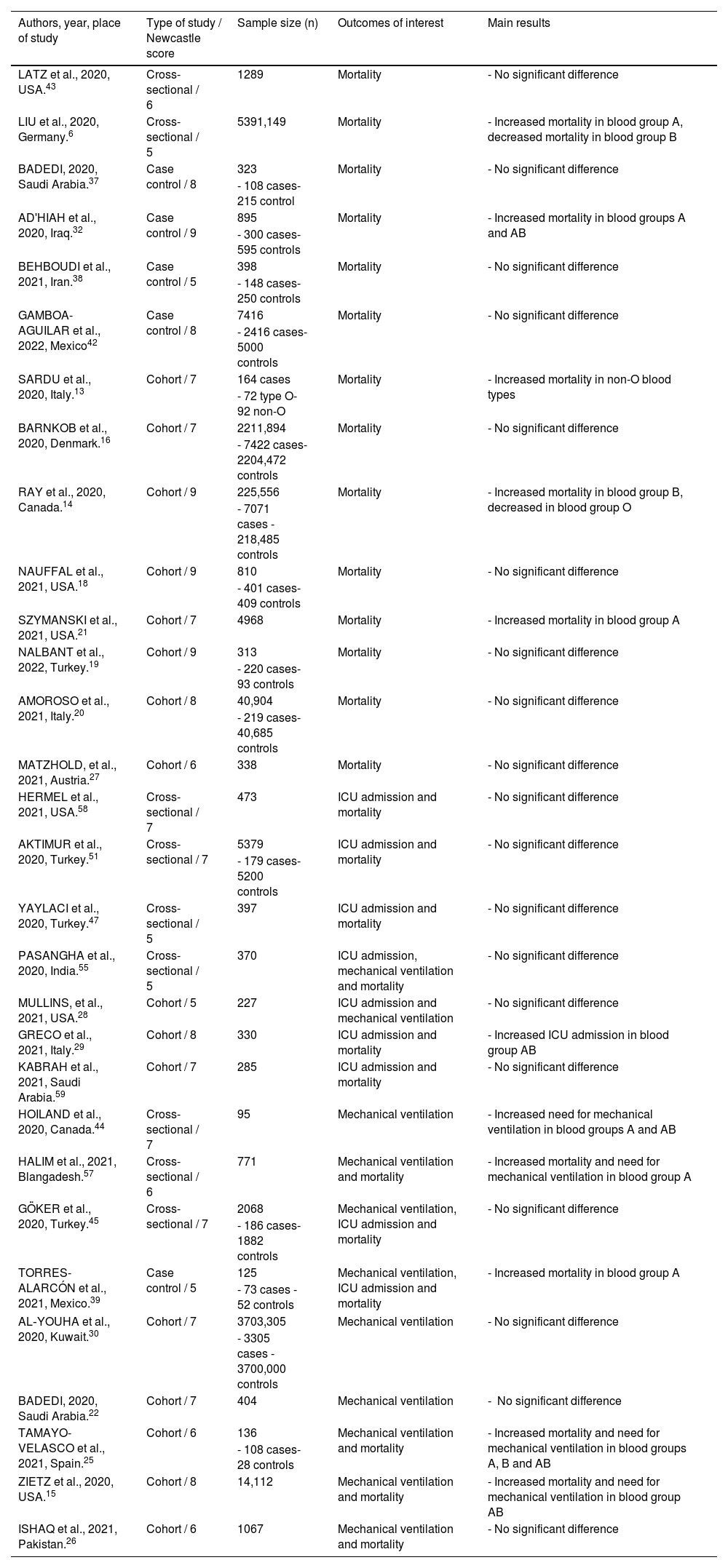
For objective 2 (COVID infection severity), 35 articles were analyzed (Table 5). Sample sizes ranged from 43 to 5391,149 participants. The studies were conducted in 17 countries and the majority were from United States (6 studies), Turkey (5 studies) and Saudi Arabia (4 studies). Regarding the type of studies found, 16 were cohort, 13, cross-sectional, and 5, case control. We assessed studies including any of the three selected outcomes of interest (mortality, ICU and mechanical ventilation).
Studies that show correlation between ABO blood group and COVID severe infection.
| Authors, year, place of study | Type of study / Newcastle score | Sample size (n) | Outcomes of interest | Main results |
|---|---|---|---|---|
| LATZ et al., 2020, USA.43 | Cross-sectional / 6 | 1289 | Mortality | - No significant difference |
| LIU et al., 2020, Germany.6 | Cross-sectional / 5 | 5391,149 | Mortality | - Increased mortality in blood group A, decreased mortality in blood group B |
| BADEDI, 2020, Saudi Arabia.37 | Case control / 8 | 323 | Mortality | - No significant difference |
| - 108 cases- 215 control | ||||
| AD'HIAH et al., 2020, Iraq.32 | Case control / 9 | 895 | Mortality | - Increased mortality in blood groups A and AB |
| - 300 cases- 595 controls | ||||
| BEHBOUDI et al., 2021, Iran.38 | Case control / 5 | 398 | Mortality | - No significant difference |
| - 148 cases- 250 controls | ||||
| GAMBOA-AGUILAR et al., 2022, Mexico42 | Case control / 8 | 7416 | Mortality | - No significant difference |
| - 2416 cases- 5000 controls | ||||
| SARDU et al., 2020, Italy.13 | Cohort / 7 | 164 cases | Mortality | - Increased mortality in non-O blood types |
| - 72 type O- 92 non-O | ||||
| BARNKOB et al., 2020, Denmark.16 | Cohort / 7 | 2211,894 | Mortality | - No significant difference |
| - 7422 cases- 2204,472 controls | ||||
| RAY et al., 2020, Canada.14 | Cohort / 9 | 225,556 | Mortality | - Increased mortality in blood group B, decreased in blood group O |
| - 7071 cases - 218,485 controls | ||||
| NAUFFAL et al., 2021, USA.18 | Cohort / 9 | 810 | Mortality | - No significant difference |
| - 401 cases- 409 controls | ||||
| SZYMANSKI et al., 2021, USA.21 | Cohort / 7 | 4968 | Mortality | - Increased mortality in blood group A |
| NALBANT et al., 2022, Turkey.19 | Cohort / 9 | 313 | Mortality | - No significant difference |
| - 220 cases- 93 controls | ||||
| AMOROSO et al., 2021, Italy.20 | Cohort / 8 | 40,904 | Mortality | - No significant difference |
| - 219 cases- 40,685 controls | ||||
| MATZHOLD, et al., 2021, Austria.27 | Cohort / 6 | 338 | Mortality | - No significant difference |
| HERMEL et al., 2021, USA.58 | Cross-sectional / 7 | 473 | ICU admission and mortality | - No significant difference |
| AKTIMUR et al., 2020, Turkey.51 | Cross-sectional / 7 | 5379 | ICU admission and mortality | - No significant difference |
| - 179 cases- 5200 controls | ||||
| YAYLACI et al., 2020, Turkey.47 | Cross-sectional / 5 | 397 | ICU admission and mortality | - No significant difference |
| PASANGHA et al., 2020, India.55 | Cross-sectional / 5 | 370 | ICU admission, mechanical ventilation and mortality | - No significant difference |
| MULLINS, et al., 2021, USA.28 | Cohort / 5 | 227 | ICU admission and mechanical ventilation | - No significant difference |
| GRECO et al., 2021, Italy.29 | Cohort / 8 | 330 | ICU admission and mortality | - Increased ICU admission in blood group AB |
| KABRAH et al., 2021, Saudi Arabia.59 | Cohort / 7 | 285 | ICU admission and mortality | - No significant difference |
| HOILAND et al., 2020, Canada.44 | Cross-sectional / 7 | 95 | Mechanical ventilation | - Increased need for mechanical ventilation in blood groups A and AB |
| HALIM et al., 2021, Blangadesh.57 | Cross-sectional / 6 | 771 | Mechanical ventilation and mortality | - Increased mortality and need for mechanical ventilation in blood group A |
| GÖKER et al., 2020, Turkey.45 | Cross-sectional / 7 | 2068 | Mechanical ventilation, ICU admission and mortality | - No significant difference |
| - 186 cases- 1882 controls | ||||
| TORRES-ALARCÓN et al., 2021, Mexico.39 | Case control / 5 | 125 | Mechanical ventilation, ICU admission and mortality | - Increased mortality in blood group A |
| - 73 cases - 52 controls | ||||
| AL‐YOUHA et al., 2020, Kuwait.30 | Cohort / 7 | 3703,305 | Mechanical ventilation | - No significant difference |
| - 3305 cases - 3700,000 controls | ||||
| BADEDI, 2020, Saudi Arabia.22 | Cohort / 7 | 404 | Mechanical ventilation | - No significant difference |
| TAMAYO-VELASCO et al., 2021, Spain.25 | Cohort / 6 | 136 | Mechanical ventilation and mortality | - Increased mortality and need for mechanical ventilation in blood groups A, B and AB |
| - 108 cases- 28 controls | ||||
| ZIETZ et al., 2020, USA.15 | Cohort / 8 | 14,112 | Mechanical ventilation and mortality | - Increased mortality and need for mechanical ventilation in blood group AB |
| ISHAQ et al., 2021, Pakistan.26 | Cohort / 6 | 1067 | Mechanical ventilation and mortality | - No significant difference |
The mortality was evaluated in 30 studies. Seven studies found increased mortality in blood group A patients, 2, in blood group B patients, and 4, in blood group AB patients. One study also found reduced mortality in blood group O. The majority (21 studies) found no significant difference in mortality among ABO blood groups.
Admission to the ICU was evaluated in 10 studies. Most (9 studies) found no significant correlation between the ABO blood group and need for ICU admission. One study (with 330 patients) found an increased need for ICU admission in blood group AB patients.
Mechanical ventilation was analyzed in 10 studies. Most (6 studies) also found no significant difference between the ABO blood group and need for mechanical ventilation. Three studies found an increased need for mechanical ventilation in blood group A patients, 3, in blood group AB patients, and 1, in blood group B patients.
Analyzing all three severity outcomes, the majority of the studies found no significant difference in either mortality, ICU admission or need for mechanical ventilation associated with the ABO blood group in COVID-19 patients. Only 12 of the 35 studies found a significant correlation, mostly showing an increased mortality and need for mechanical ventilation in blood group A patients.
DiscussionWe performed a systematic review of the correlation between the ABO blood type and susceptibility to COVID-19 infection, both in terms of risk of infection and in terms of disease severity. The results of this review point to a possible increased risk of COVID-19 infection associated particularly with blood type A and a possible decreased risk of infection in blood type O, as most high-quality studies including either outpatients or hospitalized patients and with blood donors or non-blood donors as controls replicated this finding.
The relationship between the ABO blood group and risk of COVID-19 infection has been previously investigated by other review studies.6-9 Most reviews showed similar findings of an increased risk in group A patients and a decreased risk in group O. However, the present review includes a larger number of studies and more recently published papers, which might better reflect the present phase of the COVID-19 disease pandemic.
As for disease severity, results were conflicting, as most studies found no statistically significant correlations. Those that have found differences mostly reported increased mortality in group A patients. Admission to the ICU was mostly similar among ABO blood groups in all except one study and the need for mechanical ventilation was higher in blood group A and B in only 3 in 11 studies.4,5
Previously published reviews also bring conflicting results on this subject. One review has found increased mortality in group A versus non-A.6 Another study found lower risk of death in ABO group B patients.8 However, most reviews failed to demonstrate an increased mortality associated with any ABO blood group,4,9 similar to the findings of the present review, albeit with fewer articles included in the analysis. A definitive association of the ABO blood group distribution with mortality or severity outcomes remains to be proven.
Studies utilizing blood donors as controls carry a potential risk of bias, as the frequency of ABO blood group subtypes may be different between blood donors and the general population.1,2 Studies with blood transfusions could also influence outcomes due to different profiles between donors and transfusion recipients. One study by Muñiz-Diaz et al. (2020) reported 2 different samples, one with blood donor controls and another with non-blood donor controls and found different results, namely a significant difference (increased A and decreased O blood groups in patients) when comparing patients to blood donor controls and no significant difference when comparing transfusion recipients with COVID-19 infection to recipients without COVID-19 infection (non-blood donor controls).5 However, when summarizing all studies with blood donor controls and those with non-blood donor controls, results were largely similar (an increased risk in blood group A patients and a decreased risk in blood group O patients in most studies).
Patient assessment methods varied among studies. We only extracted data regarding the ABO group distribution among infected and non-infected subjects for objective 1 (the risk of COVID-19 infection). For objective 2 (the severe COVID-19 infection), we extracted data regarding mortality, admission to the ICU and the need for mechanical ventilation for analysis, regardless of other clinical outcomes evaluated by the selected studies. This was an attempt to draw more homogenous conclusions based on heterogenous studies.
The mechanisms responsible for this correlation between ABO blood groups and the susceptibility to COVID-19 infection are still unclear. Guillon et al. (2008) demonstrated that viral S proteins co-localize with the ABO group A antigens and that anti-A antibodies could inhibit the adhesion between the S protein and host cell ACE2 receptors that initiate viral invasion.10 This could explain why type O patients (who have both anti-A and anti-B antibodies) might be protected from infection and also why type A patients (who do not possess anti-A antibodies) might be at greater risk. Deleers et al. (2021) reported that COVID-19-infected patients had significantly lower levels of anti-ABO antibodies, reinforcing the hypothesis that these antibodies might have a protective role. Indeed, lower susceptibility to infection in group O patients has been previously reported for SARS-CoV, another member of the Coronavirus family.11
Another hypothesis to explain a relationship between the ABO blood group and COVID-19 disease outcomes involves the risk of thromboembolic complications. Group O individuals have lower levels of pro-thrombotic von Willebrand factor (vWF) and may be partially protected against COVID-associated thrombosis, an important mechanism in many complications leading to worse outcomes.12 This is in line with the findings from Ladikou et al. (2020) and Sardu et al. (2020), included in the present review, which have shown higher levels of vWF in non-O patients associated with worse outcomes.12,13
The present study has several limitations. We have found high heterogeneity among the studies, regarding different populations, study designs, sample sizes, control groups, follow-up periods and outcome measures. For this reason, we did not perform a meta-analysis of the results. Limiting the analysis to less heterogeneous studies with a low risk for bias might have reduced sample size significantly. Also, the studies analyzed lacked important information, such as participant vaccination status and SARS-CoV2 variant reported, which might impact the outcomes. Due to the time in which our assessment was performed, most patients were not infected with the Omicron variant, that is presently the dominant variant worldwide. Results with the Omicron variant, including BA.1 and BA.2 variants, might have been different.
This review summarizes all available studies to date regarding the association between the ABO blood group and susceptibility to COVID-19 infection and disease severity. A rigorous methodology was used to analyze and synthesize data and the risk for bias and quality of evidence were systematically assessed. Many previous reviews were published in an earlier time during the pandemic and thus, included fewer studies. Another strength of our review is that we only included studies with laboratory-confirmed SARS-CoV-2 infection. We have also tried to approach the susceptibility to infection and disease severity as different objectives, selecting studies that utilized these specific outcomes for each analysis.
Understanding patient susceptibility to COVID-19 infection according to the ABO blood group could help us in identifying new risk factors to be incorporated in prognostic tools and triage algorithms. Comprehension of the mechanisms by which this susceptibility is manifested might also open new treatment possibilities by providing novel therapeutic targets or utilizing naturally occurring protective factors associated with ABO blood group antigens or antibodies.
Future studies specifying the vaccination status and identifying particular SARS-CoV-2 variants, especially the dominant Omicron variants, and the susceptibility of these patients according to the ABO blood group might further optimize the COVID-19 risk stratification and treatment.
ConclusionQualitative assessment of available information suggests that the blood group A may be a risk factor for the COVID-19 infection and that the blood group O may have a protective effect. Based on all evidence available at this point, we were unable to determine a clear association between the ABO blood group and COVID-19 associated mortality. These conclusions were based on highly heterogenous evidence and furthermore, contain several biases. Moreover, they do not reflect the predominance of omicron variants and of vaccinated subjects, which characterize the present moment of the pandemic. Further studies are needed to assess the actual impact of the ABO blood group on the risk and severity of the COVID-19 infection.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Availability of data and materialsNot applicable.
FundingNot applicable.
Credit authorship contribution statementDMBS, DABSA, PRN, JLBS and DABSA contributed to the article conception and wrote the manuscript; DMBS, DABSA, RBM, EMBS, FCSN, MSNP and VCVG contributed to the data collection and analysis. DMBS, DABSA, MSNP, PRN, PBN and GFA read, revised and approved the final version of the manuscript.
We offer our thanks to the Study Group on Neuroinflammation and Neurotoxicology at the State University of Ceará (GENIT/UECE).
The authors are grateful to the Brazilian National Council for Scientific and Technological Development (CNPq) for the funding of the Productivity scholarship to the author Pedro Braga Neto and to the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) for the funding of Pedro Braga Neto (88881.505364/2020-01).