The treatment of multiple myeloma (MM) has evolved significantly in the past decade, and new drug combinations have improved the response rates and prolonged survival. Studies comparing different induction chemotherapy regimens have shown that triple combinations have better results than double combinations. However, comparisons among different triple combinations are rare in the literature.
MethodsWe retrospectively compared two triple combinations comprising bortezomib, cyclophosphamide and dexamethasone (VCD) versus thalidomide, cyclophosphamide and dexamethasone (CTD), and aimed at identifying which of the two combinations would yield better response rates following four induction cycles prior to hematopoietic cell transplantation in patients with untreated multiple myeloma.
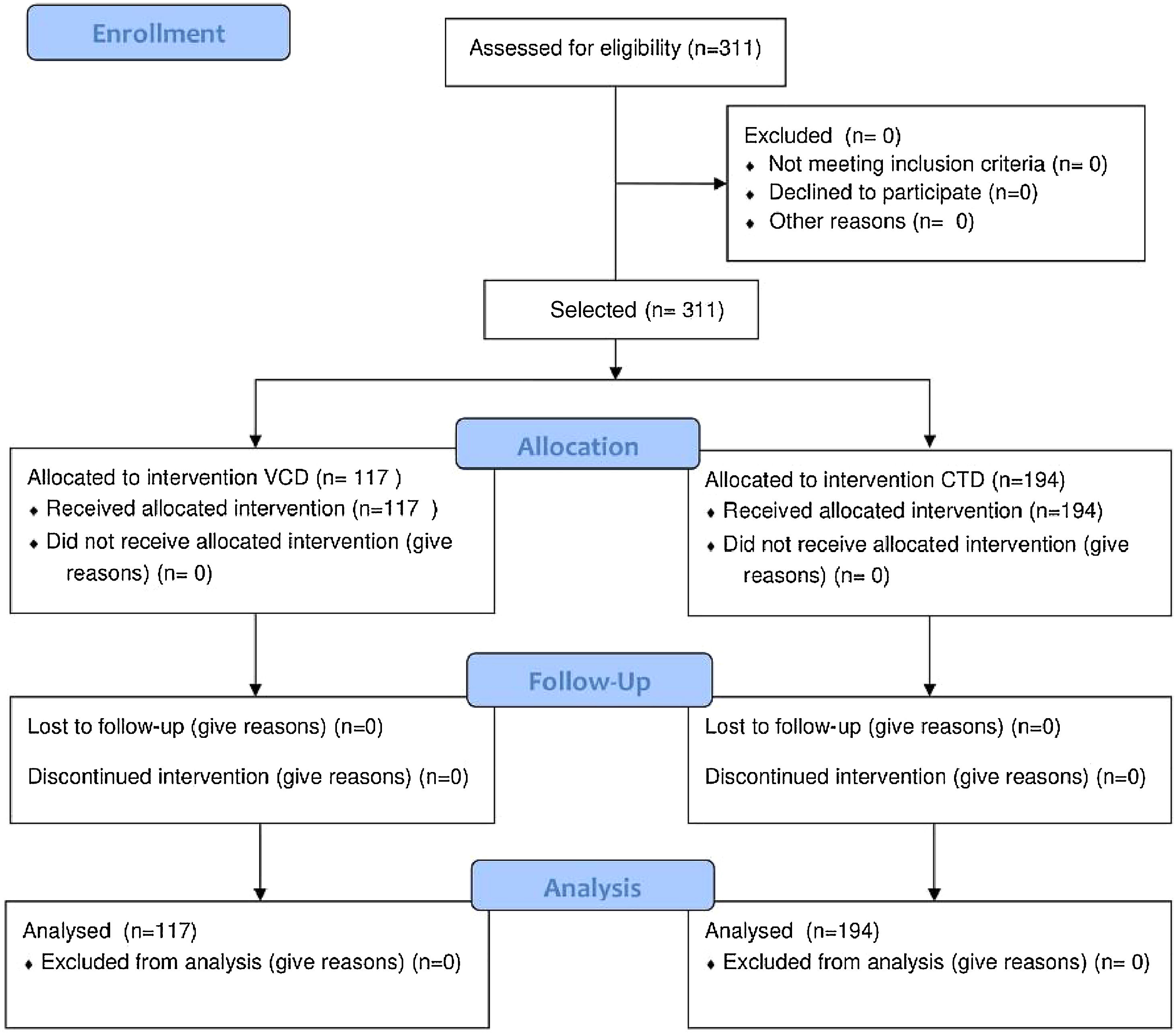
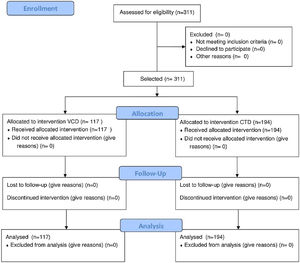
ResultsWe retrospectively reviewed the medical records of 311 patients from 24 different centers.The VCD regimen was used as induction therapy by 117 (37.6%) patients, whereas 194 (62.4%) patients received the CTD regimen. After four cycles of induction on an intention-to-treat basis, 54% of the patients in the VCD group achieved at least very good partial response versus 42.8% in the CTD group (p=0.05). We observed no difference in neuropathy or thrombotic events rates among the two regimens.
ConclusionOur results corroborate the superiority of the triple combination regimes containing bortezomib over the triple combination with thalidomide as pre ASCT induction therapy in MM.
Multiple myeloma (MM) is the second most common hematologic neoplasm.1 Its treatment has been evolving over the years with a significant increase in response to therapy and survival rates.1 The various combinations of novel agents as immunomodulatory agents (IMIDs) and proteasome inhibitors significantly reduced tumor burden prior to, and following, high doses of chemotherapy and consequently increased rates of deep remissions.2–8 Achieving better responses prior to autologous hematopoietic stem cell transplantation (ASCT) is one of the predictors of longer survival and constitutes an important goal in the treatment of MM patients.9 Three-drug induction regimens such as thalidomide, adriamycin, and dexamethasone (TAD); bortezomib, adriamycin, and dexamethasone (PAD); bortezomib, thalidomide, and dexamethasone (VTD); bortezomib, cyclophosphamide, and dexamethasone (VCD); and bortezomib, lenalidomide, and dexamethasone (VRD) demonstrated superiority over two-drug combinations.3,4,7,10,11 However, studies with new drugs comparing different triples combinations before ASCT are scarce.8,12 A non-bortezomib-containing combination with adequate response rates before ASCT was cyclophosphamide, thalidomide, and dexamethasone (CTD).5 The most commonly used regimens in Brazil as ASCT induction to eligible MM patients are CTD and VCD. In the present study, we retrospectively compared these two triplet combination regimens in order to evaluate if there is any difference in response rates after four cycles of induction treatment.
MethodsThis is a retrospective multicenter study that included a total of 311 newly diagnosed MM patients, as based on the International Myeloma Working Group (IMWG) diagnostic criteria.13 The patients were transplant candidates and underwent treatment with a VCD or CTD regimen protocol between June 2009 and June 2014.
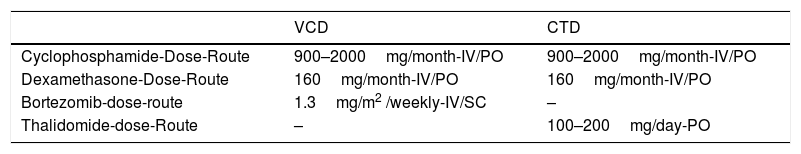
TreatmentThe CTD regimen entailed using oral or venous cyclophosphamide, continuous oral thalidomide, and oral dexamethasone.The VCD combination comprised venous or subcutaneous bortezomib, oral or venous cyclophosphamide, and oral or venous dexamethasone. The drug administration route depended on the protocol used by each center. Drug doses are shown in Table 1. The patients in the CTD group underwent venous thromboembolism (VTE) prophylaxis with 100mg/day of acetylsalicylic acid. Response assessment was carried out after four cycles of induction treatment according to the IMWG guidelines, which considers the following response categories: stringent complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD), and progressive disease (PD).14 A minimal response (MR) category was also used, as defined by the European Bone Marrow Transplantation criteria.13 The response assessment evaluation was made by each treatment center and the revision was centralized for the study analyses.
Dose and application routes in the different treatment protocols.
| VCD | CTD | |
|---|---|---|
| Cyclophosphamide-Dose-Route | 900–2000mg/month-IV/PO | 900–2000mg/month-IV/PO |
| Dexamethasone-Dose-Route | 160mg/month-IV/PO | 160mg/month-IV/PO |
| Bortezomib-dose-route | 1.3mg/m2 /weekly-IV/SC | – |
| Thalidomide-dose-Route | – | 100–200mg/day-PO |
VCD: bortezomib, cyclophosphamide and dexamethasone; CTD: cyclophosphamide, thalidomide and dexamethasone; IV: intravenous; VO: oral.
The main objective of the present study was to identify the rates of better responses (>VGPR) after four cycles of induction chemotherapy. The data collected from patient records comprised demographic characteristics, and other data of interest, including gender, age, Durie-Salmon (DS) staging, International Staging System (ISS), paraprotein type, performance status, presence of bone lesions, type of patient center type (public or private care center), and presence of comorbidities (high blood pressure, diabetes mellitus, or liver disease). Toxicity data (peripheral neuropathy and thrombosis) were also evaluated. The patients were analyzed based on the group to which they had been initially assigned (VCD or CTD), according to the intention-to-treat (ITT) principle. The Kolmogorov–Smirnov test was used to evaluate the distribution pattern of numeric variables within the sample. The unpaired t-test was used to compare the means of variables with a normal distribution, whereas the Mann–Whitney test was used to compare ages between groups. Categorical variables, including response rates, were compared using chi-square or Fisher's exact tests, as appropriate. For ordinal categorical variables with more than two categories, the chi-square test was used for trends. Statistical significance levels were considered between the groups when p<0.05. Statistical analyses were performed with the MedCalc software package (Mariakerke, Belgium, v. 11.3.3.0). The study protocol was approved by the research ethics committees at each institution. Clinical Trial: NCT03402295.
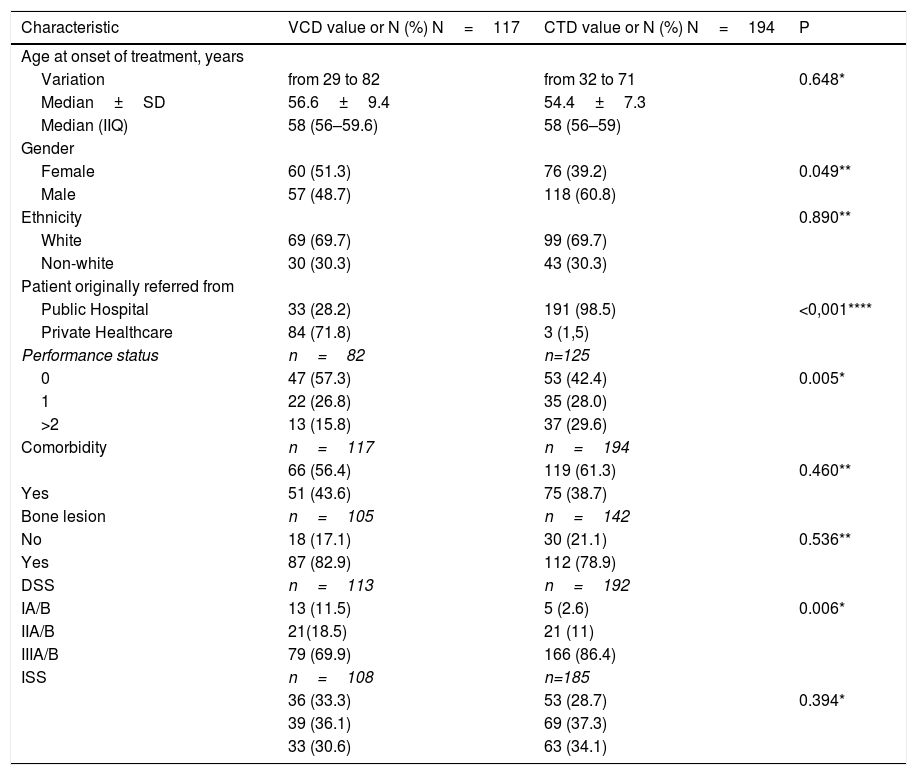
ResultsA total of 311 patients were included in this analysis. The VCD regimen was used as induction therapy by 117 (37.6%) patients, whereas 194 (62.4%) patients received CTD (Figure 1). Of the 24 participant centers, eight were private centers and 16 were either public or philanthropic centers. Of the patients who received the VCD regimen, 84 (71.7%) were treated at private centers, and 33 (28.3%) were treated at public or philanthropic centers. Of the 194 patients treated with CTD, only three cases were assisted in the private system, while all others received treatment in public or philanthropic systems. The main demographic and clinical characteristics of the patients are shown in Table 2, according to the treatment group. The median age at initial treatment in the two combined groups was 58 years (51–62 years), and no differences were observed between groups, as for the epidemiological-demographic data. The performance status of the patients in the CTD group was worse when compared to that of the patients in the VCD group. Similarly, when applying the Durie and Salmon staging (DSS) system, more advanced stages were found in the CTD group. A total of fifty-five patients (17.6%), 30 on CTD and 25 on VCD, had creatinine above 2g/dL at diagnosis. There were no patients registered on dialysis.
Clinical and demographic characteristics of patients.
| Characteristic | VCD value or N (%) N=117 | CTD value or N (%) N=194 | P |
|---|---|---|---|
| Age at onset of treatment, years | |||
| Variation | from 29 to 82 | from 32 to 71 | 0.648* |
| Median±SD | 56.6±9.4 | 54.4±7.3 | |
| Median (IIQ) | 58 (56–59.6) | 58 (56–59) | |
| Gender | |||
| Female | 60 (51.3) | 76 (39.2) | 0.049** |
| Male | 57 (48.7) | 118 (60.8) | |
| Ethnicity | 0.890** | ||
| White | 69 (69.7) | 99 (69.7) | |
| Non-white | 30 (30.3) | 43 (30.3) | |
| Patient originally referred from | |||
| Public Hospital | 33 (28.2) | 191 (98.5) | <0,001**** |
| Private Healthcare | 84 (71.8) | 3 (1,5) | |
| Performance status | n=82 | n=125 | |
| 0 | 47 (57.3) | 53 (42.4) | 0.005* |
| 1 | 22 (26.8) | 35 (28.0) | |
| >2 | 13 (15.8) | 37 (29.6) | |
| Comorbidity | n=117 | n=194 | |
| 66 (56.4) | 119 (61.3) | 0.460** | |
| Yes | 51 (43.6) | 75 (38.7) | |
| Bone lesion | n=105 | n=142 | |
| No | 18 (17.1) | 30 (21.1) | 0.536** |
| Yes | 87 (82.9) | 112 (78.9) | |
| DSS | n=113 | n=192 | |
| IA/B | 13 (11.5) | 5 (2.6) | 0.006* |
| IIA/B | 21(18.5) | 21 (11) | |
| IIIA/B | 79 (69.9) | 166 (86.4) | |
| ISS | n=108 | n=185 | |
| 36 (33.3) | 53 (28.7) | 0.394* | |
| 39 (36.1) | 69 (37.3) | ||
| 33 (30.6) | 63 (34.1) |
Cyclophosphamide was used in both treatment regimens. In the CTD group oral cyclophosphamide was administered to 152 patients and intravenously to 42 patients. In the VCD group, cyclophosphamide was administered intravenously in 95 cases, orally in four cases, and in 18 patients the administration route was not recorded (Table 1). Therefore, in both treatment protocols, the final total cumulative dose of cyclophosphamide over four cycles ranged from 3600 to 8000mg. In the VCD group, a median dose of 1730mg (462–2452mg) per cycle was administered and a median of 1500mg (500–2452mg) was administered to the CTD group, thereby ensuring homogeneity for comparison among the groups.
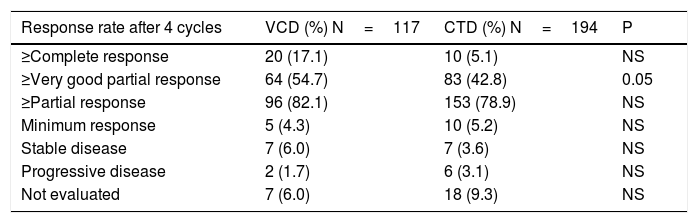
After four cycles of induction treatment VGPR or better was observed in 54.7% of the VCD group vs. 42.8% in the CTD group (p=0.05). The response rates are described in Table 3. Patients without a response assessment were considered as non-responders. An analysis correlating treatment center type (public vs. private), DSS, and performance status (PS) was carried out, including whether a response rate of at least PR was obtained or not. None of these variables could be related to response type (Table 4). In respect to neurological toxicity, peripheral neuropathy was reported in 114 cases, 46 in the VCD group (39.3%) and 68 in the CTD group (35.1%). There was no significant difference between the two groups (P=0.526).The degree of peripheral neuropathy, reported in 85 of the 114 cases, demonstrated no significant difference in distribution by degree in either group. Venous thromboembolism (VTE) was reported in only 14 cases (four patients treated with VCD and 10 patients with CTD) with no significant difference among the groups.
Response rates based on intention-to-treat between VCD and CTD groups.
| Response rate after 4 cycles | VCD (%) N=117 | CTD (%) N=194 | P |
|---|---|---|---|
| ≥Complete response | 20 (17.1) | 10 (5.1) | NS |
| ≥Very good partial response | 64 (54.7) | 83 (42.8) | 0.05 |
| ≥Partial response | 96 (82.1) | 153 (78.9) | NS |
| Minimum response | 5 (4.3) | 10 (5.2) | NS |
| Stable disease | 7 (6.0) | 7 (3.6) | NS |
| Progressive disease | 2 (1.7) | 6 (3.1) | NS |
| Not evaluated | 7 (6.0) | 18 (9.3) | NS |
VCD: velcade, cyclophosphamide, and dexamethasone; CTD: cyclophosphamide, thalidomide, and dexamethasone; ITT: intention-to-treat; NS: not significant.
Correlation between (original treatment center or performance status or DS prognosis) and response rate better than PR.
The new treatments for MM have significantly increased response rates and patient survival.15 Three-drug induction protocols for patients eligible for transplantation including new agents (thalidomide, bortezomib, and lenalidomide) have shown better responses than the double combinations.3–12 However, few studies comparing triplet combinations, including the new agents, have been published to date. The German group conducted a non inferiority study comparing triple combinations including bortezomib (VCD vs. PAD) as a pre-ASCT induction treatment. The study demonstrated no inferiority in the VCD combo. The response rates better than VGPR after three induction cycles was 37% for VCD vs. 34.3% for the PAD combo.12 Another recently published study showed VTD superiority over VCD, in respect to higher VGPR and overall response (OR) rates after four cycles of therapy prior to ASCT.8 In both studies, bortezomib was combined in different associations. In one of them, an immunomodulator was associated with one of the combinations as an attempt to demonstrate the better effectiveness of two new agents versus the classic combinations used in chemotherapy (alkylating agents and/or anthracyclines). Our study compared two new agents (thalidomide vs bortezomib) comprising different triple combinations, based on the association of cyclophosphamide and dexamethasone used as a pre-ASCT induction treatment (VCD vs CDT). Vigolo et al.16 showed in a single-center study with 43 patients that there was no difference in response rates between the VCD and CTD regimens after four cycles of induction treatment. Nevertheless, in the present multicenter study with a sample of 311 patients, the combination with VCD was found to have superior response rates better than very good partial response (>VGPR) after 4 cycles of induction treatment (p=0.05), hence suggesting the superiority of bortezomib over thalidomide with the combination of cyclophosphamide and dexamethasone. In a meta-analysis of four prospective phase III trials including 1572 patients, Sonneveld et al. found that the use of bortezomib as part of the induction treatment in patients eligible for ASCT not only was superior in terms of responses, but also yielded better survival rates.17 Another meta-analysis performed with four randomized studies including 2169 patients eligible for ASCT also found that the use of bortezomib during induction treatment significantly improved response rates, therefore directly impacting on progression-free and overall survival rates.18
One of the major adverse events observed with the use of bortezomib and/or thalidomide is peripheral neuropathy.19 Modifications in the use of bortezomib, such as a weekly and subcutaneous administration, allowed a reduction in grade 3 or higher neuropathy rates.20,21 Almost all of the cases included here were patients receiving bortezomib weekly and subcutaneously, which yielded lower peripheral neuropathy rates, comparable with those demonstrated in similar studies. Thalidomide has a neuropathic effect that relates mainly to time and dose accumulation.22 The evaluation after four cycles in the CTD regimen found neuropathy>grade 3 in about 7% of cases. In a randomized study, Morgan et al. identified neuropathy grade>3 in approximately 4% of patients.5 Another thalidomide-related adverse event is the risk of developing venous thromboembolism.19 Patients in the CTD group were invariably using 100mg/day of acetylsalicylic acid prophylactically. The VCD group has no indication for antithrombotic prophylaxis. The total number of thrombosis cases in this study was low (8.4%) and even lower in the CTD group (5%). Morgan et al.5 identified thrombosis in 15% of patients receiving a CTD regimen in a randomized study.
The present study also included a comparison between groups of patients from private and public centers. The CTD protocol was used in 98% of patients from public centers. We found a difference in performance status (PS) between public and private centers, with disadvantages for the former. This finding may be related to a delay in diagnosis and access to treatment and consequently worse clinical status. A significantly lower difference was observed in the CTD group with respect to the laboratory values of hemoglobin, ionic calcium and DHL. (data not shown) Although there were differences in staging (DSS, PS and treatment center) between the two groups, the univariate analysis did not show a significant impact between responders and non-responders. In conclusion, this analysis showed that the combination of bortezomib, cyclophosphamide, and dexamethasone (VCD) was superior to the combination of thalidomide, cyclophosphamide, and dexamethasone (CTD) with respect to the best responses after four cycles of induction treatment for MM patients eligible for ASCT.
Clinical practice points- •
To date, there has been scant information on studies comparing proteasome inhibitor (VCD) versus immunomodulator (CTD) as an induction for patients with multiple myeloma eligible for bone marrow transplantation.
- •
After four cycles of induction, an advantage was identified for better response rates than very good partial response for the VCD versus CTD group. Besides that, the toxicity was easily manageable in the VCD group.
- •
Induction protocols with proteasome inhibitor (PI)-VCD, VTD or VRD were superior when compared to other schemes for the induction of myeloma patients eligible for autologous stem cell transplantation (ASCT). In Brazil, thalidomide is available to all patients. However, PI is limited for use in patients treated by the public health system. The present study reinforces that protocols with PI are superior as for response rates and can be one more tool that supports the importance of this drug (PI) as part of induction protocols for the majority of ASCT eligible myeloma patients. This is very important for our region in which the other option is CTD, and our data highlights the inclusion of bortezomib in the treatment of the public health system myeloma patient.
None.
Conflict of interestThe authors declare no conflicts of interest.