Diversity in Classical Hematology Research
More infoThe therapeutic plasma exchange (TPE) controls the systemic cytokine level and might improve the immune response in patients with severe Coronavirus (COVID-19) infection. To date, in developing countries, no study has explored the effectiveness and risk factors in a population with severe COVID-19 exposed to the TPE.
MethodWe described the risk factors associated with survival rates higher than 28 days and length of stay (LOS) in the intensive care unit (ICU) shorter than 15 days. Severe COVID-19 cases treated with TPE were included, from August 2020 to June 2021. Survival analysis with Kaplan-Meier curves, log-rank tests and multivariate logistic regressions were conducted to assess patient-related factors that could predict a higher survival rate and the ICU LOS.
ResultsA total of 99 patients with severe COVID-19 who had received TPE were followed during their hospital stay and for 28 days after discharge. The sample was composed of men (63%) aged 56 ± 16 years. The overall survival rate at 28 days was 80%. The ICU LOS (p = 0.0165) and mechanical ventilation (MV) (p = 0.00008) were considered factors that could increase the risk of death. Patient-related factors that influenced the 28-day mortality were the smoking status (OR = 5.8; 95%CI 1.5, 22) and history of oncologic or non-malignant hematologic diseases (OR = 5.9; 95%CI 1.2, 29).
ConclusionPatients with severe COVID-19 exposed to the TPE were associated with a 20% risk of death in a 28-day observation window, appearing to be lower than previous treatments. Active smoking, cancer and immunosuppressive conditions should be considered as relevant variables to be controlled in future trials on the TPE and COVID-19.
To date (December 1, 2022), 643 million confirmed cases of Coronavirus (COVID-19) infection and more than 6.63 million associated deaths were identified worldwide, according to the World Health Organization.1
In developing countries, the impact of such a disease might have been worse than in higher income countries, due to the reduced access to the health care system, higher geographic population density, health inequalities and important social and economic disparities.2 Brazil had an incidence rate of 1160 per 10,000 habitants, while in Canada the incidence was 783 per 10,000 habitants (data from January 27, 2022).1,3 If the Brazilian healthcare system, regarding access and resources available, were similar to those in developed countries, the impact of the morbidity and mortality could have been lower.4
Despite the high number of COVID-19 trials (n > 7381) to date, experimental treatments for COVID-19 infection are still scarce.5 Indeed, many experimental approaches were offered to reduce the risk of death and to mitigate intensive care unit (ICU) admissions and prolonged stay. The mechanical ventilation (MV) use was also considered an important outcome in those trials, because of the difficulty in obtaining hospital supplies during the pandemic. In addition to dexamethasone, few therapeutic alternatives were associated with improved outcomes.6,7
One of the treatment approaches included therapeutic plasma exchange (TPE),8 a procedure that is able to downregulate prothrombotic proteins, decreasing antifibrinolytic mediators and restoring the balance of anticoagulant proteins in patients with COVID-19 infection. It is thought that the cytokine storm, mainly due to the interleukin-6 (IL-6) and IL-23, in association with the release of pro-thrombotic agents, increases the likelihood of thromboembolic events, which was seen in 21% of the patients.9
The TPE use has been raising the interest of many researchers, as 28 ongoing trials were registered in clinicaltrials.gov. However, until recently, only observational evidence suggested that the TPE might be associated with an improved overall survival (OS). In patients with COVID-19, a 28-day survival with the TPE (91%, 95%CI 78; 97) appears to be higher than the propensity score-matched controls who received the standard of care (61%, 95%CI 51; 78). In addition, the rapid reduction of pro-inflammatory cytokines might promote better oxygen saturation and the control of high levels of transaminases, creatinine, ferritin, c-reactive protein, and dimer-D.11
That said, in developing countries, such as Brazil, where the burden of COVID-19 seems to be a significant public health problem, the TPE used as an available alternative therapeutic might be an interesting resource to decrease ICU length of stay (LOS) and mortality.
In this exploratory study, we described the risk factors for ≥ 15 days of ICU stay and the 28-day mortality in severe COVID-19 cases treated with the TPE in Brazil.
MethodsStudy designIn this prospective cohort study, we described the risk factors for ≥ 15 days of ICU stay and the 28-day mortality in severe COVID-19 cases treated with TPE in Brazil from August 8, 2020 to June 2, 2021. The study was Institutional Review Board (IRB)-approved and had the consent of all patients or caregivers to receive the TPE.
Inclusion of patientsThe study included: (a) adult patients (≥ 18 years old) with the confirmation of severe COVID-19, which meant the positive PCR-RT test and ICU admission due to respiratory insufficiency (O2 > 10 L/min); (b) viral infection symptoms in the last 12 days; (c) high levels of pro-calcitonin; (d) infection-like hemogram; (e) lymphopenia, and; (f) more than 50% compromised lung seen through tomography.
ProceduresAll patients received the best ICU support, which included respiratory support (invasive or non-invasive methods), dialysis (as needed), gastric protection, corticosteroids, thromboembolism prophylaxis and symptomatic treatment.
The TPE was performed through a central venous catheter. For 3 to 5 consecutive days, patients received continuous cycles of Optia® (Terumo ©), exchanging 1 to 1½ plasma volumes (50 ml/kg) and fresh plasma replacement. In other words, some patients were submitted to 3, some to 4 and some to 5 TPE sessions. There were no data collection regarding the TPE safety, as it is well tolerated.
Statistical analysesSurvival analysis with Kaplan-Meier curves and log-rank tests were used to explore the OS, and the influence of mechanical ventilation (MV) and > a 15-day ICU stay on the 28-day mortality after hospital admission. Univariate and multivariate logistic regressions were conducted to assess patient-related factors that could predict a prolonged ICU stay (> 15 days) and 28-day mortality. Results were reported as odds ratios (ORs), 95% confidence intervals (CIs) and p-values.
Sample sizeAs the current study is exploratory, that is, it has no prespecified hypothesis testing, the sample size was calculated based on the proportion of patients with COVID-19 treated with the TPE. The Kamran et al. study suggested that 91% of the patients survived after the TPE use.11 By using a 10% margin of error, we are 95% confident that a sample of at least 32 patients would be necessary to represent the Kamran et al. case register.12 Therefore, the study tried to recruit about thrice the sample required above to reduce uncertainty.
ResultsOf the 114 patients initially eligible, 99 had a complete data collection and 15 were excluded due to the lack of data on mortality and other study outcomes.
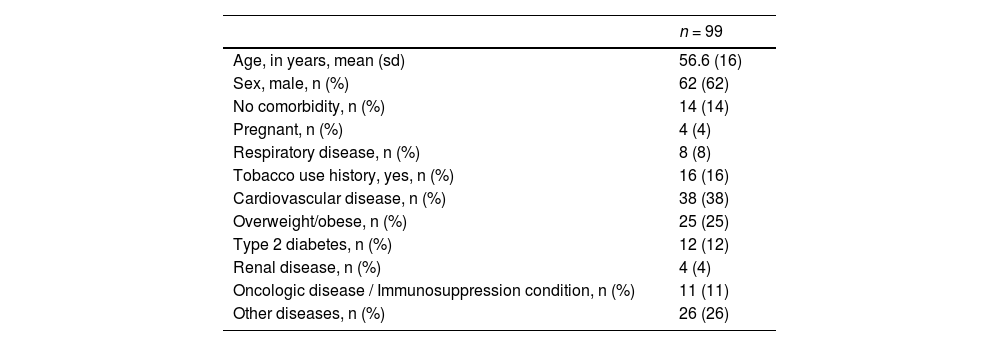
The sample was mainly composed of adults (56 years old, on average) and men (63%). Most of the patients required MV (92%) and had a history of cardiovascular disease (38%), followed by obesity (25%), smoking history (16%) and cancer or a non-malignant hematologic condition (11%) (Table 1).
Baseline characteristics of 99 patients with severe Covid-19 submitted to therapeutic plasma exchange.
| n = 99 | |
|---|---|
| Age, in years, mean (sd) | 56.6 (16) |
| Sex, male, n (%) | 62 (62) |
| No comorbidity, n (%) | 14 (14) |
| Pregnant, n (%) | 4 (4) |
| Respiratory disease, n (%) | 8 (8) |
| Tobacco use history, yes, n (%) | 16 (16) |
| Cardiovascular disease, n (%) | 38 (38) |
| Overweight/obese, n (%) | 25 (25) |
| Type 2 diabetes, n (%) | 12 (12) |
| Renal disease, n (%) | 4 (4) |
| Oncologic disease / Immunosuppression condition, n (%) | 11 (11) |
| Other diseases, n (%) | 26 (26) |
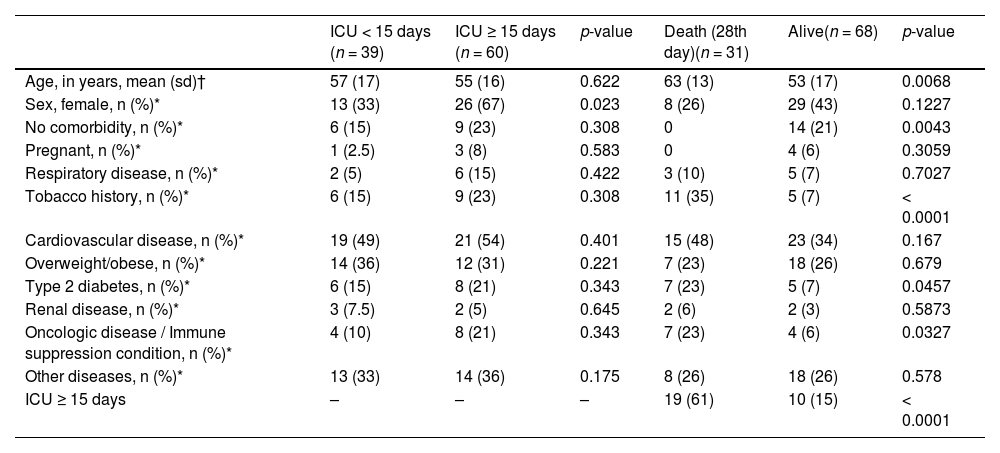
The 28-day survival rate was 80%. In the exploratory analysis, among those patients who required MV, the length of stay was higher in those who died (23 days vs. 15 days, p = NS). Survival rate was lower in patients exposed to MV (p = 0.00008). A shorter ICU stay was a significant predictor of high survival rates (≤ 15 days vs. > 15 days, p = 0.0165).
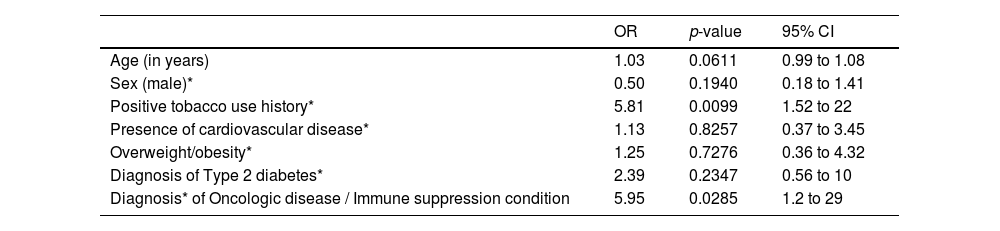
Initially, when assessing patient-related risk factors, age, female sex, absence of comorbidity, smoking history, type-2 diabetes and cancer/hematologic conditions were considered risk factors for the 28-day mortality. (Table 2) However, in multivariate analysis, only the smoking status (15%, OR = 5.8; 95%CI 1.5, 22) and history of oncologic or hematologic diseases (11%, OR = 5.9; 95%CI 1.2, 29) were shown to be robust predictors of death (Table 3).
Bivariate analyses considering baseline characteristics and ICU stay or death.
| ICU < 15 days (n = 39) | ICU ≥ 15 days (n = 60) | p-value | Death (28th day)(n = 31) | Alive(n = 68) | p-value | |
|---|---|---|---|---|---|---|
| Age, in years, mean (sd)† | 57 (17) | 55 (16) | 0.622 | 63 (13) | 53 (17) | 0.0068 |
| Sex, female, n (%)* | 13 (33) | 26 (67) | 0.023 | 8 (26) | 29 (43) | 0.1227 |
| No comorbidity, n (%)* | 6 (15) | 9 (23) | 0.308 | 0 | 14 (21) | 0.0043 |
| Pregnant, n (%)* | 1 (2.5) | 3 (8) | 0.583 | 0 | 4 (6) | 0.3059 |
| Respiratory disease, n (%)* | 2 (5) | 6 (15) | 0.422 | 3 (10) | 5 (7) | 0.7027 |
| Tobacco history, n (%)* | 6 (15) | 9 (23) | 0.308 | 11 (35) | 5 (7) | < 0.0001 |
| Cardiovascular disease, n (%)* | 19 (49) | 21 (54) | 0.401 | 15 (48) | 23 (34) | 0.167 |
| Overweight/obese, n (%)* | 14 (36) | 12 (31) | 0.221 | 7 (23) | 18 (26) | 0.679 |
| Type 2 diabetes, n (%)* | 6 (15) | 8 (21) | 0.343 | 7 (23) | 5 (7) | 0.0457 |
| Renal disease, n (%)* | 3 (7.5) | 2 (5) | 0.645 | 2 (6) | 2 (3) | 0.5873 |
| Oncologic disease / Immune suppression condition, n (%)* | 4 (10) | 8 (21) | 0.343 | 7 (23) | 4 (6) | 0.0327 |
| Other diseases, n (%)* | 13 (33) | 14 (36) | 0.175 | 8 (26) | 18 (26) | 0.578 |
| ICU ≥ 15 days | – | – | – | 19 (61) | 10 (15) | < 0.0001 |
Legends: † Mann-Whitney U; * chi-square or Fisher exact test, as appropriate.
28-day mortality predictors based on multivariate analyses.
| OR | p-value | 95% CI | |
|---|---|---|---|
| Age (in years) | 1.03 | 0.0611 | 0.99 to 1.08 |
| Sex (male)* | 0.50 | 0.1940 | 0.18 to 1.41 |
| Positive tobacco use history* | 5.81 | 0.0099 | 1.52 to 22 |
| Presence of cardiovascular disease* | 1.13 | 0.8257 | 0.37 to 3.45 |
| Overweight/obesity* | 1.25 | 0.7276 | 0.36 to 4.32 |
| Diagnosis of Type 2 diabetes* | 2.39 | 0.2347 | 0.56 to 10 |
| Diagnosis* of Oncologic disease / Immune suppression condition | 5.95 | 0.0285 | 1.2 to 29 |
Observations: *references for regression analyses respectively include the following (females), negative tobacco use history, absence of cardiovascular disease, no overweight or obesity status, no type 2 diabetes, no diagnosis of oncologic or immune suppression condition.
The first study on the TPE in critically severe COVID-19-infected patients in Brazil suggested that the 28-day mortality (20%) was similar to previous estimates.11 In a larger and comparative study conducted in Pakistan, Kamran et al., 2021, applied the same technique of TPE in a similar population that was studied in the present paper and found that survival was higher among those who received the procedure (91%, 95% CI 78.33 – 97.76) and better than the propensity score-matched population (61, 95% CI 51.29 – 78.76, log rank p-value < 0.001). Other studies have been reporting similar results in both the TPE-exposed group and controls, suggesting that the benefits of such a procedure might be valid and reproducible through different settings.13
These findings are compatible with the previous hypothesis on the cytokine storm effect on multiple-organ failure, where it appears that a strategy, such as the TPE, that interferes with as many as possible inflammatory pathways, including those related to coagulation activation, might be associated with better results.14 Immune system down regulators, such as dexamethasone, provided lower 28-day mortality in comparison to a placebo (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81).15 Several drugs that failed to demonstrate mortality reduction were commonly single cytokine regulators, such as tocilizumab (IL-6) and chloroquine (TNF and IL-6).14,16
One important issue that might impact TPE-related effectiveness is the early initiation of the TPE (1 to 1 ½ times the patient plasma volume with fresh frozen plasma), use of 4 - 5% albumin or COVID-19 convalescent plasma as replacement fluids before multiorgan failure, as they had demonstrated better COVID-19 recovery.17 However, the TPE still lacks randomized controlled trial confirmation, which is expected to come from one of the 28 ongoing studies, according to clinicaltrials.gov (last seen October 2021).10
The benchmark 28-day mortality rate for severe COVID-19 can be as high as 40% in patients who did not receive steroids and 35% in patients not exposed to early TPE.11,15 On the other hand, it is not feasible to treat all severe COVID-19 patients, as seen with other resources that became scarce during the pandemic, such as oxygen and even ICU beds. In this scenario, it might appear that treating patients at higher risk of death, such as those identified in our study (smoking history or cancer diagnosis, for example) might provide a better sense of priority when decision-making on better resource allocation is needed.
This study is not absent of limitations. Firstly, this is a cohort study that, neither aimed to test the hypothesis that the TPE is better than the standard ICU care, nor looked at determining the adverse event rates related to the TPE, though no catheter-related events were found in the study. Even if the prolonged ICU stay were associated with lower survival rates, it does not mean that the TPE was an independent predictor of the ICU stay. Further comparative studies (parallel-arm investigations between two or more therapies) are needed to conclude that the TPE might reduce the ICU length of stay. On the other hand, our findings were similar to larger studies in different settings, suggesting that there might be an external validity in our findings. That said, local experience with the TPE could be considered successful and should be validated through experimental study designs. Secondly, we did not investigate the role of cytokine levels, ICU scores (such as the APACHE) and results might be limited to the investigated covariates. Therefore, independent predictors of mortality identified in this paper should be carefully interpreted. It is important to comment that COVID-19 still is a condition with few studies. As an example, the APACHE scores are not able to completely predict survival in severely infected patients18. Finally, though limitations are presented in the study, this paper has a significant contribution to public health, as COVID-19 was one of the most impacting infectious diseases in human history. It is also highly relevant when science still failed to demonstrate a quick response to identify therapies that could recover patients from COVID-19 infections. Identifying therapies that could reduce the clinical burden of COVID-19 does not mean that prevention (vaccine and public isolation) is not needed. On the contrary, new therapies might be relevant to improve resource allocation (prevent the COVID-19 prolonged hospital stay, for example) in budget constrained health systems.
ConclusionIn this exploratory study, patients with severe COVID-19 exposed to the TPE were associated with a 20% risk of death in a 28-day post-discharge follow-up, appearing to be lower than the risk rates in previous treatments. The smoking status and history of cancer or hematologic diseases might predict an increased risk of death, which might be relevant variables to be controlled in ongoing trials on the TPE.