To compare the effect of aquatic and land-based physiotherapy in reducing musculoskeletal hip and lower back pain and increasing overall physical capabilities of sickle cell disease patients.
MethodsInformed written consent was obtained from all volunteers who were submitted to evaluations using different functional scales: Lequesne's Algofunctional Questionnaire and Oswestry Disability Index, trunk and hip range of motion, goniometry, trunk and hip muscle strength assessment using load cell, and surface electromyography of the iliocostalis, long dorsal (longissimus), gluteus maximus, gluteus medius and tensor fasciae latae muscles. Ten patients were randomized into two groups: aquatic physiotherapy with a mean age of 42 years (range: 25–67) and conventional physiotherapy with a mean age of 49 years (range: 43–59). Both groups were submitted to a twelve-week program of two sessions weekly.
ResultsAfter the intervention, significant improvements were observed regarding the Lequesne index (p-value=0.0217), Oswestry Disability Index (p-value=0.0112), range of motion of trunk extension (p-value=0.0320), trunk flexion muscle strength (p-value=0.0459), hip extension and abduction muscle strength (p-value=0.0062 and p-value=0.0257, respectively). Range of motion of trunk and hip flexion, extension, adduction and abduction, trunk extensor muscle strength and all surface electromyography variables showed no significant statistical difference.
ConclusionPhysical therapy is efficient to treat musculoskeletal dysfunctions in sickle cell disease patients, irrespective of the technique; however, aquatic therapy showed a trend toward improvement in muscle strength. Further studies with a larger patient sample and longer periods of therapy are necessary to confirm these results.
Sickle cell disease (SCD) is a genetic disorder that results in the sickling of red blood cells, triggering vaso-occlusion episodes which lead to pain and organ damage. This inherited disorder is caused by a point mutation in the beta-globin gene. The mutant form of hemoglobin in SCD (Hb S) is capable of polymerization and complex molecular and structural changes within the red cell. Occurring in homozygotes (Hb SS) and in compound heterozygotes, such as those carrying Hb S and beta-thalassemia (Hb SB+ or Hb SB0) and Hb S and hemoglobin C (Hb SC), hemolytic anemia and vaso-occlusion crises are the main complications of SCD. The illness tends to gradually develop toward multisystem organ failure.1 Bone involvement, frequently causing painful vaso-occlusive crises, is the most common clinical manifestation. Furthermore, bone involvement is a source of chronic, progressive disability, with long-term effects upon bone mass density, growth, and bone damage such as avascular necrosis and osteomyelitis. Osteopenia and osteoporosis are often asymptomatic; however, pain, fractures, deformities, and vertebral collapse may occur and require chronic analgesia, mechanical support, and surgical interventions.2,3
Chronic and progressive damage such as, for example, avascular necrosis of the femoral head, the leading cause of hip deformity in these patients, commonly results in gait disturbances, pain, and activity and functional limitations in adult patients. Lower back pain is one of the main complaints among SCD patients and occurs due to the flattening and widening of the vertebral bodies with biconcave depressions of the endplates, probably caused by infarction of the central portion of the vertebral body.4,5 There are few studies in the literature on the role of physiotherapy as a resource to prevent and treat locomotor system disorders in SCD individuals.
According to recent studies, the life expectancy of SCD patients’ has improved dramatically over the last century.6 However this longer life span has, as an unfortunate consequence, the development of progressive organ damage which includes osteoarticular lesions.7
Chronic pain is considered a serious public health problem which negatively affects the quality of life of individuals. Therefore, a multi-action therapeutic plan, specifically physiotherapy, could help decrease pain, and improve mobility and the rehabilitation of osteoarticular disorders, positively impacting on the quality of life.8
Despite this fact, there are few studies in the literature on the role of physiotherapy as a resource to prevent and treat locomotor system disorders in SCD patients. One study9 compared the efficacy of physiotherapy alone with physiotherapy associated with surgical femur decompression in SCD patients with osteonecrosis of the femoral head. The results showed no significant difference between these two approaches, suggesting that physical therapy alone appeared to be as effective as surgical decompression to improve hip function, thus deferring the need for surgery.
Within the existing physiotherapy resources, aquatic physiotherapy used in rehabilitation has demonstrated positive effects against pain, in regaining physical function and in improving quality of life in adults with musculoskeletal conditions.10 Movements performed in the water are facilitated by the elimination of the effects of gravity, resulting in increased muscle strength (MS) and flexibility. The benefits of water are mainly explained by the physiological effects of immersion and by the hydrodynamic principles of exercise, such as buoyancy, in this environment thereby enabling functional exercises with a reduced gravitational load. Furthermore, the immersion in thermo-neutral water (34°C) in combination with the effects of hydrostatic pressure reduces the perception of pain. The physical properties and heated water play an important role in improving and maintaining the range of joint motions, reducing muscular tension and promoting relaxation, as well as preparing the muscle for stretching. The buoyancy induces muscle relaxation and the decrease in impact enables increased mobility and flexibility.11,12
This study aimed to evaluate the efficacy of aquatic and land-based physical therapy in decreasing hip and lower back musculoskeletal pain and increasing overall physical wellbeing in SCD patients.
MethodsAdult SCD patients who regularly attended (at least three times a year during the previous three years) the Outpatient Clinic of the Hemocentro of the Universidade Estadual de Campinas (UNICAMP) with chronic hip and lumbar spine pain, and who had not participated in a physical therapy program during the previous 12 months, were invited to participate in this study. Patients with acute episodes, absence of over three physical therapy sessions without justification, or any dermatological issue which would prevent them from entering a therapeutic pool, were excluded from the study. The National Ethics Board approved this study, and all patients provided written informed consent.
Study designInitially, the volunteers were evaluated according to functional scales, including the Lequesne's Algofunctional Questionnaire and Oswestry Disability Index (ODI), range of motion (RoM) measurements of trunk flexion and extension, hip adduction and abduction, assessment of MS of the trunk flexors and extensors, and the flexors, extensors, adductors and abductors of the hip through load cell and surface electromyography (SEMG) of the iliocostalis, long dorsal (longissimus), gluteus maximus, gluteus medius and tensor fasciae latae muscles. Volunteers were then randomized by an investigator not involved in data collection, using the blind allocation method of sequentially numbered, opaque sealed envelopes,13,14 into two different program groups: aquatic physiotherapy (AP) and conventional or land physiotherapy (CP). A total of 24 sessions over a twelve-week period (two sessions per week) were administered. Patients were assessed after the intervention comparing the results before and after the sessions according to data obtained for the dominant side of each patient.15,16
QuestionnairesThe Lequesne's Algofunctional Questionnaire was developed for patients with osteoarthritis and evaluates symptoms and functional capacity of the hip and knee. This index is composed of 11 questions that evaluate pain, discomfort and function. The ODI is a self-administered questionnaire used to measure the degree of lumbar spine disability, and contains topics concerning intensity of pain and physical activity.17 The ODI has been used in scientific research to evaluate patients with nonspecific or specific low-back pain after surgical procedures, medication and rehabilitation.
Range of motionThe RoM was evaluated by a single examiner using a conventional 360° free shaft goniometer. The following movements were assessed: trunk flexion and extension and flexion, extension, adduction and abduction of the hip joint, according to the standardization of the goniometry manual of Marques.18
Muscle strengthMS was analyzed by maximal voluntary isometric contraction (MVIC) using a load cell (MIOTEC®, Porto Alegre, Brazil). The load cell was connected to a Miotool 400® apparatus (MIOTEC®) using a SDS1000® sensor connected via a USB cable to a notebook. During movements, the force generated by traction on the load cell was transmitted to the Miograph® software which produces a plot of MS in kilograms-force (kgf). Volunteers were submitted to isometric MS tests of the trunk flexors and extensors and hip flexors, extensors, adductors and abductors.
Surface electromyographyMyoelectric signals of the gluteus maximus, gluteus medius, tensor fascia lata, long dorsal (longissimus) and iliocostalis muscles were sampled at 2000Hz in single differential mode from each muscle through a four channel electromyography system (MIOTEC®, Porto Alegre, Brazil) using disposable Ag/AgCI circular bipolar electrodes (3M®). The 10mm diameter electrodes with adhesive conducting gel were positioned on the skin overlying the muscles at an inter-electrode distance of 20mm. Abrasion of the skin was achieved at the fixation sites with gauze soaked in alcohol to reduce impedance and the electrodes were then fixed at the muscular belly, distant from the motor point, and fixed with transparent tape and elastic band wrapping to avoid movement artifacts. The data acquisition Miograph USB® software system with windowing 32 (RMS – Root Mean Square) and gain of 2000 for each channel was used to capture the electrical potentials of the muscles evaluated in microvolts (μV). Butterworth filters were used: order 4 and band pass 20–500Hz. The four channels were connected to active SDS500® sensors by clamps. Signal analysis was performed using Miograph USB® system software. The sensors were calibrated before data collection. The electrical potentials of the muscles were collected in accordance to international standardization of SENIAM.19
Aquatic physiotherapyThe 9m2 pool in a 16m2 room was warmed to 34°C; the patients changed their clothes in this temperature-controlled room. Each session consisted of lower limb muscle stretching, jogging in the pool (forward, backward and sideways), suspended bicycle exercises in the vertical position, stair climbing exercises, active exercises in the supine position using floats, and finally relaxation exercises.
Conventional physiotherapyEach session consisted of lower limb stretches, hip exercises to strengthen hip adductors and abductors, supine bridge, exercises using ankle-weights to strengthen the quadriceps and when necessary, transcutaneous electrical nerve stimulation was used for pain relief.
Statistical analysesThe statistical analysis system (SAS) computer program for Windows (version 9.2) and GraphPad Prism (version 5.00 – Trial) were used for statistical analysis. A p-value of 0.05 or less was considered statistically significant. The following tests were then performed.
Fisher's exact test to compare proportions, the Mann–Whitney test to compare numerical measurements between the two groups, ANOVA to compare numerical values over time with repeated measurements with transformation stations and the Wilcoxon test for paired samples before and after the intervention.
ResultsThe final sample comprised ten volunteers randomized into two groups: AP and CP. Median age was 42 years old (range: 25–67) for the AP group and 49 years old (range: 43–59) for the CP group. The clinical and laboratory data of the participants are shown in Table 1.
Clinical and laboratory data of sickle cell disease patients submitted to two physiotherapy programs.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Aquatic | Aquatic | Aquatic | Aquatic | Aquatic | Land | Land | Land | Land | Land |
| Age (years) | 42 | 25 | 67 | 28 | 53 | 59 | 49 | 45 | 58 | 43 |
| Gender | Female | Female | Male | Male | Female | Female | Female | Male | Male | Female |
| Type of disease | Hb SS | Hb SS | Hb SC | Hb SS | Hb SC | Hb SC | Hb SS | Hb SC | Hb SC | Hb SC |
| Transfusions | No | Yes | No | No | Yes | No | No | No | No | No |
| α-Thalassemia | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | H |
| Leukocytes (×109/L) | 6.13 | 8.41 | 6.80 | 6.04 | 7.49 | 10.88 | 13.61 | 4.44 | 5.97 | 6.34 |
| Hemoglobin (g/dL) | 7.4 | 7.1 | 10.4 | 10.1 | 9.6 | 10.2 | 10.6 | 11.7 | 12.7 | 10.0 |
| MCV (fL) | 113.7 | 101.0 | 67.0 | 104.7 | 91.9 | 82.4 | 85.4 | 76.9 | 77.2 | 69.3 |
| Reticulocytes (%) | 12.68 | 3.51 | 3.59 | 11.81 | 7.48 | 7.56 | 2.70 | 4.83 | 5.55 | 4.90 |
| Platelet count (×109/L) | 331 | 335 | 508 | 304 | 392 | 163 | 175 | 94 | 275 | 114 |
| Fetal hemoglobin (%) | 7.3 | 4.0 | 0.2 | 23.7 | 2.8 | 1.4 | 28.1 | 0.5 | 1.0 | 1.2 |
| LDH (U/L) | 1680 | 944 | 405 | 1000 | 535 | 445 | 862 | 403 | 529 | 431 |
| HU dose (mg/kg) | 10 | 11 | 0 | 26 | 0 | 0 | 21 | 0 | 0 | 0 |
MCV: mean corpuscular volume; LDH: lactate dehydrogenase; HU: hydroxyurea; Neg: negative.
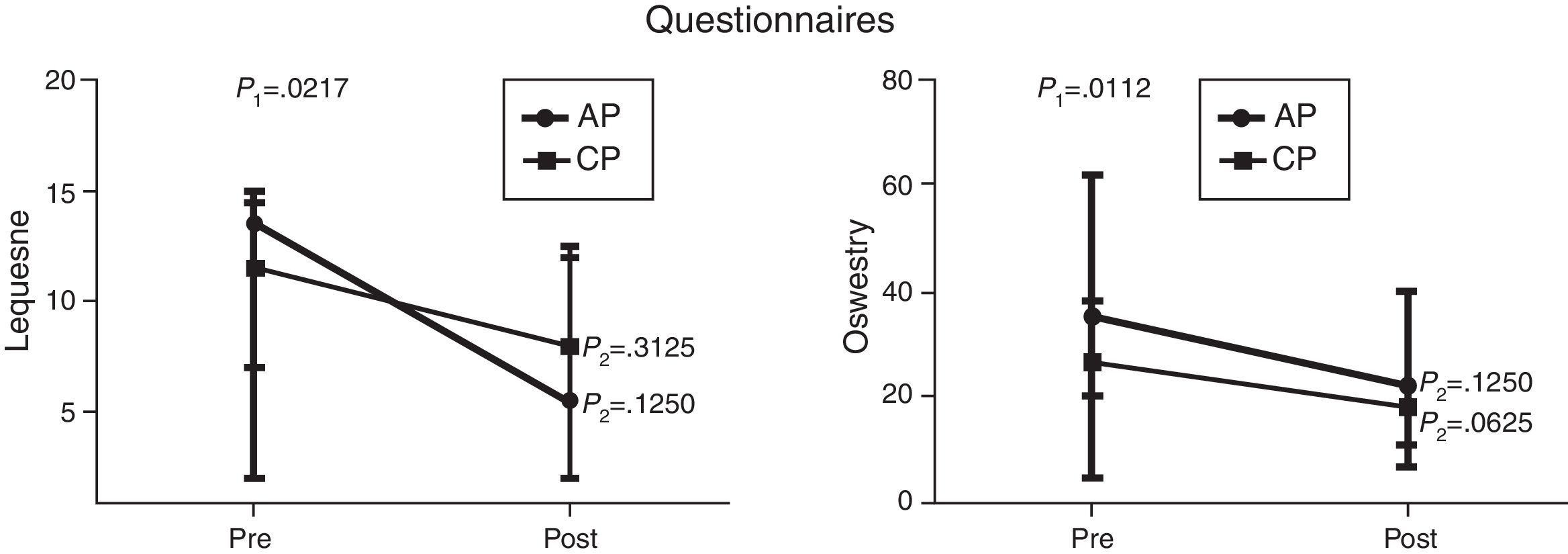
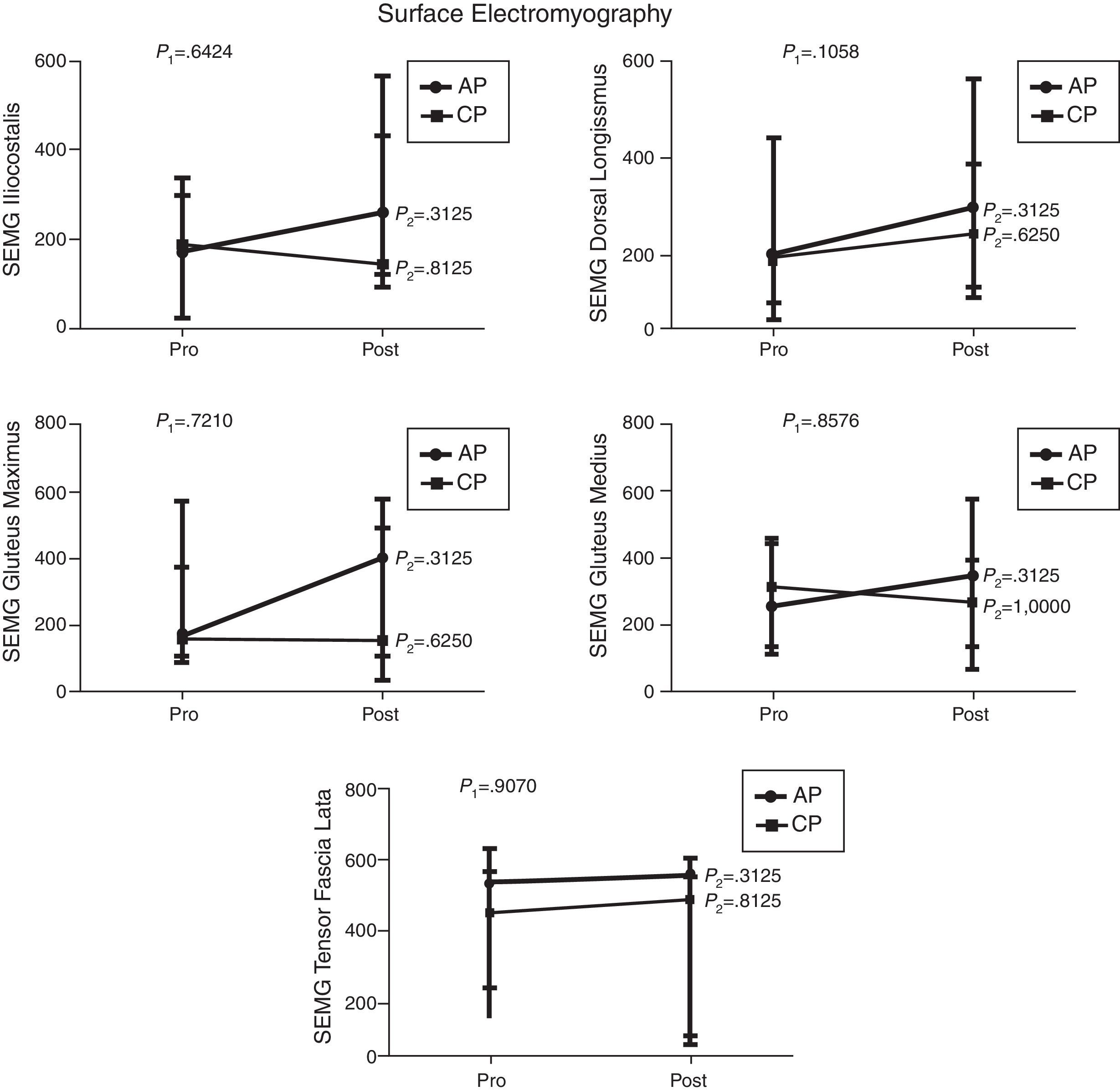
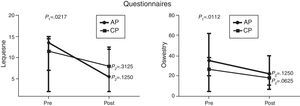
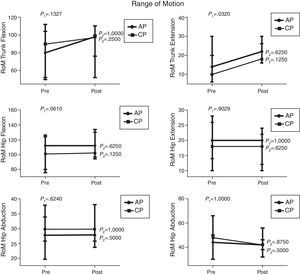
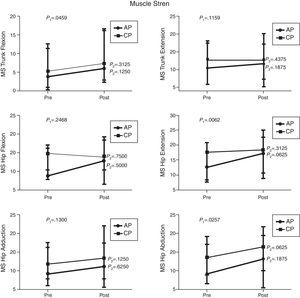
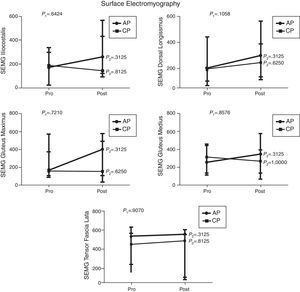
Comparison of numerical values over time between the two groups showed a statistically significant difference after the intervention in respect to the Lequesne index (p-value=0.0217), ODI (p-value=0.0112), RoM of trunk extension (p-value=0.0320), trunk flexion MS (p-value=0.0459), and hip extension and abduction MS (p-value=0.0062 and p-value=0.0257, respectively). There were no significant statistical differences in the RoM of trunk and hip flexion, extension, adduction and abduction, trunk extensor MS and hip flexion and adduction MS and all SEMG variables (Figures 1–4).
Median, minimum and maximum values of the Lequesne index (Lequesne's Algofunctional Questionnaire) and Oswestry Disability Index evaluated before and after interventions for the aquatic physiotherapy (AP) and conventional physiotherapy (CP) groups. The p1-value refers to the comparison of numerical values over time with repeated measurements with transformation stations (ANOVA test). The p2-value refers to the comparison between time points for each group (Wilcoxon test).
Median, minimum and maximum values of Range of Motion (RoM) of truck flexion and extension and hip flexion, extension, adduction and abduction measured by goniometry. Variables evaluated before and after interventions for the aquatic physiotherapy (AP) and conventional physiotherapy (CP) groups. The p1-value refers to the comparison of numerical values over time with repeated measurements with transformation stations (ANOVA test). The p2-value refers to the comparison between time points for each group (Wilcoxon test).
Median, minimum and maximum values of muscle strength (MS) of the trunk flexors and extensors, and hip flexors, extensors, adductors and abductors by load cell. Variables evaluated before and after the interventions for the aquatic physiotherapy (AP) and conventional physiotherapy (CP) groups. The p1-value refers to the comparison of numerical values over time with repeated measurements with transformation stations (ANOVA test). The p2-value refers to the comparison between time points for each group (Wilcoxon test).
Median, minimum and maximum values of surface electromyography (SEMG) of iliocostalis, long dorsal (longissimus), gluteus maximus, gluteus medius and tensor fasciae latae muscles. Variables evaluated before and after interventions for the aquatic physiotherapy (AP) and conventional physiotherapy (CP) groups. The p1-value refers to the comparison of numerical values over time with repeated measurements with transformation stations (ANOVA test). The p2-value refers to the comparison between time points for each group (Wilcoxon test).
The present study aimed to evaluate two types of physiotherapy intervention for hip and lumbar spine functionality of adult SCD patients. The dominant side of each patient was considered in the results.15,16
The major limitation of this study was the recruitment of volunteers, as most of the patients live far from the center and find it very difficult to attend the clinic twice every week. Therefore, only ten patients, six compound heterozygous for Hb S and Hb C and four Hb S homozygotes, completed the physiotherapeutic program.
In view of the high frequency of avascular necrosis of the femoral head in SCD patients, the intervention was focused on the hip joint. The Lequesne questionnaire was used to evaluate the functionality of this joint. This scale assesses pain and hip function for daily activities. The results of the Lequesne Algofunctional questionnaire showed a statistically significant improvement after the intervention for both the groups. The AP group had very severe impairment (13.5 points) in the first assessment and moderate impairment (5.5 points) in the second. The CP group also had very severe impairment (11.5 points) in the first assessment and severe impairment (8 points) in the second. These results suggest that aquatic physiotherapy may lead to a greater improvement in hip functionality and are in agreement with Wang et al.20 and Hinman et al.21 who observed improvement in physical function after a program of aquatic physical therapy for patients with hip and knee osteoarthritis. Other studies carried out in individuals with hip and/or knee disorders however, showed no significant differences between the two rehabilitation strategies, suggesting that both techniques are equally effective.22–24
Lower back pain is one of the main complaints of SCD patients. The ODI was herein used to assess lower back pain and function during daily activities. This index also showed statistically significant improvements in both study groups after the intervention. The CP group improved from moderate disability (26.5%) to minimal disability (18%); the AP group, however, despite some significant improvement in the scores of the second assessment (from 35% to 22%) showed no change in the severity of the disability caused by back pain. Longer or more frequent sessions may render better results, as has been described by others.24,25 These studies showed that patients who performed AP two or more times weekly had greater improvement in physical assessment scores than those who exercised only once a week.24,25 Nevertheless, Ariyoshi et al.25 extended the program for six months and concluded that water therapy exercises were useful for patients with back pain as they provide pain relief.
In this study, a significant improvement in RoM of the trunk extension and a trend toward an improvement in trunk flexion goniometry were observed in both groups, especially in the AP group. However, no significant change was detected in either group regarding motion amplitude, probably due to the inflammatory phenomena and bone infarctions which may have caused permanent limitations.
Furthermore, late interventions may not be sufficient to improve joint RoM in this age group in which chronic degenerative hip injuries may have reached a level of severity that precludes greater joint flexibility.
Moreover, the fact that there was no significant improvement in goniometry may be a consequence of the techniques used in both groups which may favor strength gain. Thus, perhaps the program should increase the time devoted to stretching certain target muscles during therapy, thereby promoting improved muscle flexibility.
Regarding trunk MS, a significant improvement in flexion was observed in both groups after the interventions. Despite the trend toward improvement in trunk extensor MS in the AP group, there were no significant differences between the two groups. These results are in agreement with other studies that showed improved MS after specific land-based and water-based trunk exercises.24,26,27
There was a statistically significant increase in hip extension and abduction MS in both groups after the interventions. Although, hip flexion MS was unchanged in the CP group, there was a trend toward improvement in the AP group, and hip adduction MS showed a slight trend toward improvement in both groups.
Thus, albeit slight, the results of this study showed improvement of all MS variables in the AP group, in accordance with Wang et al.20 who also observed improved flexibility and lower limb strength after 12 weeks of aquatic therapy, and Cochrane et al.28 who observed significant improvement in pain and physical function after aquatic exercises in adults with hip and knee osteoarthritis. In another study, Rahmann29 demonstrated a positive effect of a specific program of aquatic physical therapy on early recovery of strength after hip and knee surgeries. Furthermore, Hinman et al.21 observed a slight improvement in pain, physical function, quality of life and MS after aquatic therapy for patients with hip and knee osteoarthritis in a protocol of two sessions per week for six weeks. However, Jigami23 concluded that both programs, land-based and water-based, even when the exercises were performed only once a week, improved overall physical activity and MS in the lower limbs of osteoarthritis patients.
Surface electromyography did not show any significant difference after the interventions of both groups. However, there seemed to be an improvement in the electromyography signal of all muscles evaluated in the AP group. The better result obtained in this group may be related to the greater amount of muscle fibers recruited in aquatic therapy added to the physical properties of water such as buoyancy and multidirectional strength. These findings are in agreement with the results reported by Kaneda et al.30 who observed greater electromyography activity of all muscle movements performed in the water with floating devices.
The results of this study should be analyzed with caution as the sample size may have been a limiting factor and therefore, further studies are needed to confirm these results.
ConclusionThe results obtained here suggest that physical therapy is a resource capable of treating musculoskeletal dysfunction in SCD patients regardless of the technique used. However, exercises designed to stretch tone and strengthen the core and limb muscles carried out in the water require greater stabilization of the muscles and may justify the trend toward the better results obtained.
FundingThis work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The Hemocentro, UNICAMP, is a part of Instituto Nacional de Ciência e Tecnologia do Sangue, Brazil (INCT do Sangue – CNPq/MCT/FAPESP).
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Prof. Margareth Castro Ozelo for kindly making the facilities at the Hemophilia Unit available; Dr. Marina Pereira Colella for helping recruit the patients, Márcia Matta, Janaína Bosso Ricciardi and Glenda Feldberg Andrade Pinto for their technical assistance; and Raquel Foglio for the editing and English revision.