This study aimed to describe and compare the nutritional status of adult patients submitted to allogeneic hematopoietic stem cell transplantation at two different time points (admission and discharge).
MethodsA retrospective, descriptive and quantitative study was performed based on clinical, laboratory and nutritional data obtained from medical records of adult patients of both genders submitted to allogeneic hematopoietic stem cell transplantation in a bone marrow transplantation reference center in Rio de Janeiro in the period from 2010 to 2013. Statistical analysis was performed using the SPSS software (version 22.0).
ResultsSixty-four patients were evaluated. The mean age was 42.1±3.2 years and the most prevalent disease was acute myeloid leukemia (39%). There was a high prevalence of gastrointestinal symptoms including nausea (100%), vomiting (97%) and mucositis (93%). Between admission and discharge there was a significant decrease in the median weight (−2.5kg; 71.5 vs. 68.75kg; p-value<0.001), body mass index (−0.9kg/m2; 24.8 vs. 24.4kg/m2; p-value<0.001), and serum albumin levels (−0.2g/dL; 3.7 vs. 3.6g/dL; p-value=0.024). The survival time after hematopoietic stem cell transplantation correlated negatively with C-reactive protein at discharge (CC=−0.72; p-value<0.001) and positively with serum albumin levels (CC=0.56; p-value=0.004) and with high total protein level at discharge (CC=0.53; p-value=0.006).
ConclusionOur results suggest that patients submitted to allogeneic hematopoietic stem cell transplantation have compromised nutritional status during the hospital stay for transplantation.
Hematopoietic stem cell transplantation (HSCT) is a highly complex procedure indicated for the treatment of various diseases, including aggressive hematological, oncohematological, and genetic diseases and autoimmune disorders. HSCT involves an intravenous infusion of hematopoietic stem cells (HSC) used to restore bone marrow function. Allogeneic HSCT uses hematopoietic stem cells (HSC) either from related or unrelated donors.1,2
The conditioning regimen is intended to eradicate residual disease of the patient and, in the case of allogeneic HSCT, to induce immunosuppression to allow engraftment of the infused HSC.3,4
The aggressive conditioning regimen used for allogeneic HSCT may combine chemotherapy with total body irradiation (TBI). A large number of clinical complications is associated with this type of transplantation; the morbidity and mortality are mainly associated to opportunistic infections, graft versus host disease (GVHD), organ failure, graft failure or rejection and relapse of the underlying disease.5,6
Patients submitted to allogeneic HSCT should be considered at nutritional risk7–9 due to reduced energy intake, impaired absorption of nutrients and increased metabolic demands.10
The adverse effects of chemotherapy and TBI affect, in particular, the gastrointestinal (GI) tract and the immune system.7 Accordingly, in addition to symptoms of nausea and vomiting, severe mucositis associated with intense odynophagia, abdominal pain and diarrhea often occurs.11,12 GI complications of the conditioning regimen may last for up to 21 days after transplantation.8,10
HSC transplant patients are likely to develop a series of metabolic disorders of varying severity, mostly during the immediate post-transplant period. The main causes are the adverse effects of the conditioning regimen itself, the immunosuppressive drugs (to control or for prophylaxis of GVHD) and the total parenteral nutrition (TPN), which may increase the risk of opportunistic infections and inflammatory processes.13
GVHD is one of the most important clinical complications related to allogeneic HSCT. It is related to an immune response triggered by the donor HSC cells against the host tissues which usually involves the liver, skin and the GI tract. Acute diarrhea in the post-transplant period is usually associated with infections and GVHD.8,12,14
The present study aimed to compare the nutritional status of adult allogeneic HSCT patients at two different time points (hospital admission and discharge) and describe the prevalence of GI symptoms, the occurrence of GVHD and deaths up to 100 days after transplantation (D+100).
MethodsStudy design and populationThis was a retrospective, descriptive and quantitative study based on clinical, and laboratory data and a review of the nutritional status of patients treated in a bone marrow transplantation referral center in the city of Rio de Janeiro, Brazil. Data were obtained from medical records from August 2010 to May 2013.
Patients aged 18 and older, of both genders, with neoplastic or non-neoplastic diseases submitted to allogeneic HSCT (related or unrelated) were included in the study. Patients with previous history of HSCT and those whose medical records were unavailable were excluded from the study.
Of the 76 patients who underwent allogeneic HSCT during the study period, twelve were excluded from the study, seven due to prior HSCT and five due to the lack of medical records. Thus the study sample consisted of 64 patients.
Anthropometric parameters and laboratory data collected are for two different time points, admission to the inpatient unit for transplantation (T1) and unit discharge (T2). Clinical parameters were collected from admission until 100 days after transplant (D+100). Data on the food intake and GI symptoms refer to the hospitalization period.
The study was approved by the Ethics Committee of the bone marrow transplantation referral center.
The following clinical data were investigated: diagnosis of the underlying disease, comorbidities, type of transplant (related or unrelated), source of HSC (bone marrow, peripheral blood or umbilical cord blood), conditioning regimen, length of hospitalization, time to engraftment, GI symptoms, use of enteral nutrition (EN) and TPN, occurrence of GVHD and death. Data on age, gender, education, ethnicity and lifestyle (smoking and drinking) were also collected.
Laboratory tests included blood sugar, albumin, creatinine, hemoglobin, hematocrit, potassium, phosphorus, magnesium, total bilirubin, direct bilirubin and C-reactive protein (CRP) levels.
Moreover, anthropometric evaluations consisted of the following indicators: current weight, usual weight, height, percentage of weight loss and BMI. Nutritional status was classified according to the BMI as: severe malnutrition (<16kg/m2), moderate malnutrition (16–16.9kg/m2), mild malnutrition (17–18.49kg/m2), normal weight (18.5–24.9kg/m2), overweight (25–29.9kg/m2), mildly obese (30–34.9kg/m2), moderately obese (35–39.9kg/m2) or severely obese (>39.9kg/m2).15 The percentage of weight loss was calculated from the hospital discharge weight and the admission weight.
The food intake was classified as low (<60%), partial (from 60% to 99%) or full (100%) based on the patient's reported intake on medical records. The classification used is the one standardized by the Department of Nutrition and Dietetics at the Bone Marrow Transplantation center. Nausea, vomiting, mucositis (Grades I to IV), odynophagia, hyporexia, diarrhea, among other GI symptoms reported by patients during hospitalization were recorded.
Statistical analysisDescriptive analysis was performed, using the mean, median and standard deviation for continuous variables and frequencies for categorical variables. The Spearman correlation was used to identify associations between nutritional status and the clinical parameters of HSCT. The paired t-test was used for comparisons between the time points (admission and discharge) for variables with normal distribution, and the Wilcoxon signed-rank test and the Sign test were used for non-parametric variables. The Statistical Program for Social Sciences (SPSS version 22.0) was used for the statistical analyses. The level of significance was set at 5% for all statistical tests.
ResultsSocioeconomic characteristicsThe mean age of the patients was 42.1±3.2 years, with 8% of elderly (>60 years), and the distribution between males and females was equal (50%). The percentage of smokers was 17.7% and 12.9% consumed alcohol. Skin color was predominantly white (46%) and brown (46%) and in terms of schooling 31% of patients had completed high school and 28% had university degrees; there were no illiterate individuals in the sample.
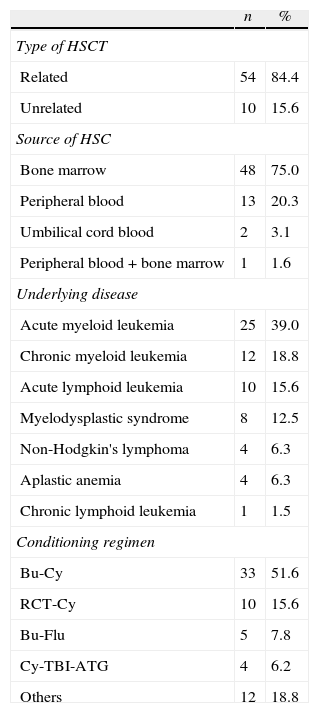
Clinical characteristics of patientsFifty-four patients (84.4%) underwent related and ten patients (15.6%) unrelated allogeneic HSCT with the main source of CTH being bone marrow (75%). The most common underlying diseases were acute myeloid leukemia (39%) and chronic myeloid leukemia (18.8%). There were prevalences of diabetes mellitus (6%) and hypertension (13%).
The main conditioning regimens and other clinical data are shown in Table 1. The mean hospital stay was 36±10 days. The mean time to engraftment was 18.3±6.6 days. The mortality rate by D+100 was 34.4% (22 patients), with 50% of the deaths occurring during hospitalization.
Clinical data and conditioning regimens.
| n | % | |
| Type of HSCT | ||
| Related | 54 | 84.4 |
| Unrelated | 10 | 15.6 |
| Source of HSC | ||
| Bone marrow | 48 | 75.0 |
| Peripheral blood | 13 | 20.3 |
| Umbilical cord blood | 2 | 3.1 |
| Peripheral blood+bone marrow | 1 | 1.6 |
| Underlying disease | ||
| Acute myeloid leukemia | 25 | 39.0 |
| Chronic myeloid leukemia | 12 | 18.8 |
| Acute lymphoid leukemia | 10 | 15.6 |
| Myelodysplastic syndrome | 8 | 12.5 |
| Non-Hodgkin's lymphoma | 4 | 6.3 |
| Aplastic anemia | 4 | 6.3 |
| Chronic lymphoid leukemia | 1 | 1.5 |
| Conditioning regimen | ||
| Bu-Cy | 33 | 51.6 |
| RCT-Cy | 10 | 15.6 |
| Bu-Flu | 5 | 7.8 |
| Cy-TBI-ATG | 4 | 6.2 |
| Others | 12 | 18.8 |
Bu: busulfan; Cy: cyclophosphamide; TBI: total body irradiation; Flu: fludarabine; ATG: anti-thymocyte globulin; Mel: melphalan; Others: Bu-Cy-ATG, Cy-ATG, Flu-Mel, Flu-Cy, Flu-ATG-Cy, Bu-Mel, Flu-Bu-ATG, Cy.
Of the 64 patients evaluated, 34 (53%) had GVHD before D+100. Among the patients with GVHD, the distribution according to the organ involved was: skin (88%), GI tract (73.5%), liver (47%); 11 patients (32%) had GVHD in three organs (skin, gastrointestinal tract and liver).
Nutritional assessmentOn admission, the nutritional assessment according to the BMI showed that 29 patients had normal weight (45.3%), 23 were overweight (35.9%), eight mildly obese (12.5%), two moderately obese (3.1%), one had mild malnutrition (1.6%) and one had moderate malnutrition (1.6%). Moreover, at this time point, 10.4% of patients had suffered a weight loss>5% of usual weight. At discharge there was a further weight loss>5% compared to the weight at admission in 40.4% of cases.
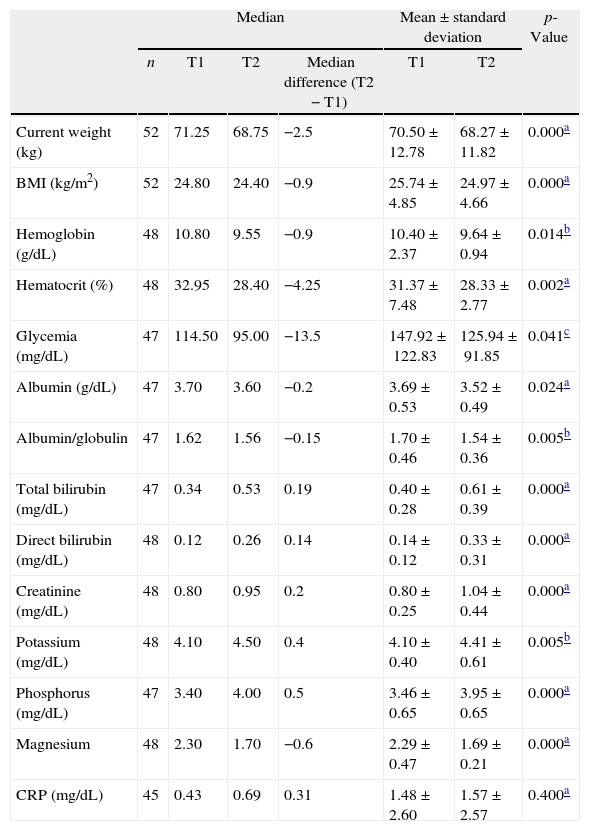
Comparing the nutritional status of patients on admission and at discharge (T1 vs. T2) the Wilcoxon signed-rank test showed that there was a statistically significant decrease in the median weight (−2.5kg; 71.5 vs. 68.75kg; p-value<0.001), BMI (−0.9kg/m2; 24.8 vs. 24.4kg/m2; p-value<0.001), and serum albumin level (−0.2g/dL; 3.7 vs. 3.6g/dL; p-value=0.024) (Table 2).
Comparison between anthropometric and laboratory variables of patients submitted to allogeneic HSCT at admission (T1) and at discharge (T2).
| Median | Mean±standard deviation | p-Value | |||||
| n | T1 | T2 | Median difference (T2−T1) | T1 | T2 | ||
| Current weight (kg) | 52 | 71.25 | 68.75 | −2.5 | 70.50±12.78 | 68.27±11.82 | 0.000a |
| BMI (kg/m2) | 52 | 24.80 | 24.40 | −0.9 | 25.74±4.85 | 24.97±4.66 | 0.000a |
| Hemoglobin (g/dL) | 48 | 10.80 | 9.55 | −0.9 | 10.40±2.37 | 9.64±0.94 | 0.014b |
| Hematocrit (%) | 48 | 32.95 | 28.40 | −4.25 | 31.37±7.48 | 28.33±2.77 | 0.002a |
| Glycemia (mg/dL) | 47 | 114.50 | 95.00 | −13.5 | 147.92±122.83 | 125.94±91.85 | 0.041c |
| Albumin (g/dL) | 47 | 3.70 | 3.60 | −0.2 | 3.69±0.53 | 3.52±0.49 | 0.024a |
| Albumin/globulin | 47 | 1.62 | 1.56 | −0.15 | 1.70±0.46 | 1.54±0.36 | 0.005b |
| Total bilirubin (mg/dL) | 47 | 0.34 | 0.53 | 0.19 | 0.40±0.28 | 0.61±0.39 | 0.000a |
| Direct bilirubin (mg/dL) | 48 | 0.12 | 0.26 | 0.14 | 0.14±0.12 | 0.33±0.31 | 0.000a |
| Creatinine (mg/dL) | 48 | 0.80 | 0.95 | 0.2 | 0.80±0.25 | 1.04±0.44 | 0.000a |
| Potassium (mg/dL) | 48 | 4.10 | 4.50 | 0.4 | 4.10±0.40 | 4.41±0.61 | 0.005b |
| Phosphorus (mg/dL) | 47 | 3.40 | 4.00 | 0.5 | 3.46±0.65 | 3.95±0.65 | 0.000a |
| Magnesium | 48 | 2.30 | 1.70 | −0.6 | 2.29±0.47 | 1.69±0.21 | 0.000a |
| CRP (mg/dL) | 45 | 0.43 | 0.69 | 0.31 | 1.48±2.60 | 1.57±2.57 | 0.400a |
T1: admission; T2: discharge; BMI: body mass index; CRP: C-reactive protein.
The serum albumin level at discharge was positively correlated with survival time after HSCT (CC=0.56; p-value=0.004). The serum total protein level at discharge also showed a positive correlation with survival time after HSCT (CC=0.53; p-value=0.006). On the other hand, the C-reactive protein level at discharge showed a strong negative correlation with survival time after HSCT (CC=−0.72; p-value<0.001).
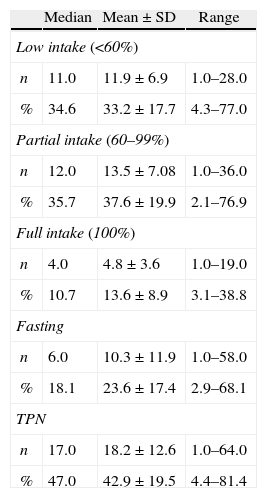
Table 3 shows the characteristics of food intake and the percentage of days of orally accepted food (low, partial or full), fasting and TPN in relation to the total length of hospitalization.
Characterization of food intake in days and percentage of hospital stay.
| Median | Mean±SD | Range | |
| Low intake (<60%) | |||
| n | 11.0 | 11.9±6.9 | 1.0–28.0 |
| % | 34.6 | 33.2±17.7 | 4.3–77.0 |
| Partial intake (60–99%) | |||
| n | 12.0 | 13.5±7.08 | 1.0–36.0 |
| % | 35.7 | 37.6±19.9 | 2.1–76.9 |
| Full intake (100%) | |||
| n | 4.0 | 4.8±3.6 | 1.0–19.0 |
| % | 10.7 | 13.6±8.9 | 3.1–38.8 |
| Fasting | |||
| n | 6.0 | 10.3±11.9 | 1.0–58.0 |
| % | 18.1 | 23.6±17.4 | 2.9–68.1 |
| TPN | |||
| n | 17.0 | 18.2±12.6 | 1.0–64.0 |
| % | 47.0 | 42.9±19.5 | 4.4–81.4 |
SD: standard deviation; TPN: total parenteral nutrition.
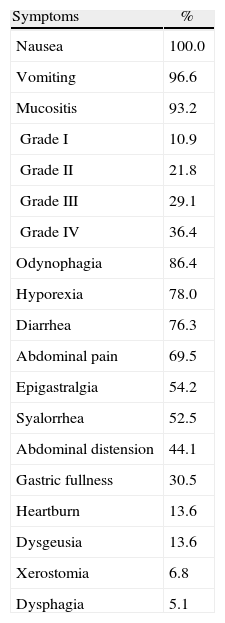
The distribution of GI symptoms reported by patients during hospitalization is shown in Table 4.
Gastrointestinal symptoms during hospitalization.
| Symptoms | % |
| Nausea | 100.0 |
| Vomiting | 96.6 |
| Mucositis | 93.2 |
| Grade I | 10.9 |
| Grade II | 21.8 |
| Grade III | 29.1 |
| Grade IV | 36.4 |
| Odynophagia | 86.4 |
| Hyporexia | 78.0 |
| Diarrhea | 76.3 |
| Abdominal pain | 69.5 |
| Epigastralgia | 54.2 |
| Syalorrhea | 52.5 |
| Abdominal distension | 44.1 |
| Gastric fullness | 30.5 |
| Heartburn | 13.6 |
| Dysgeusia | 13.6 |
| Xerostomia | 6.8 |
| Dysphagia | 5.1 |
This study in addition to describing the nutritional profile of allogeneic HSCT patients also compared the nutritional status of patients at admission and at discharge. Impaired nutritional status is considered a negative prognostic factor in hospitalized patients and is associated with adverse clinical consequences.7,10
The mean length of hospitalization observed in this study was similar to those previously reported by Bechard et al. (38 days)16 and Sommacal et al. (39 days).17 The median time to engraftment in the current study was also similar to that observed by Bechard et al. (20 days).
The mortality rate up to D+100 in this study was 34%, higher than that observed by Lee et al., 13 who reported 11.5% of deaths until D+100 in a sample of 315 allogeneic HSCT patients.
Complications related to the transplantation include the toxicity related to the conditioning regimen, the medications used to control symptoms, infections and immunosuppression, and also related to GVHD. In allogeneic HSCT, the serious side effects, including nausea, vomiting, mucositis, diarrhea and hyporexia, impair food intake; these side effects can last for up to four weeks after HSCT.18,19
The frequency of acute toxicity related to HSCT, in this context represented by GI symptoms, was high however the high aggressiveness of allogeneic transplantation should be considered. In this study, over 90% of the sample had at least three symptoms of high nutritional impact (nausea – 100%, vomiting – 96.6%, and mucositis – 93.2%). With regard to the severity of mucositis, 36% of patients developed Grade IV mucositis, thus precluding the use of oral feeding.
Dietary intake was affected greatly as can be seen by the high percentage of days of fasting (23.6±17.4%) and TPN (42.9±19.5%) and the low acceptance of food during a third of the hospital stay. Several studies have reported that food intake is significantly compromised during the period of transplantation mainly because of the side effects related to the conditioning regimen.19
The food intake of patients throughout the hospital stay was probably influenced by GI symptoms (Table 4). The mean number of days with full food intake was only 13.6% of the hospitalization period.
Among the patients studied, 62.7% received TPN, with a median of 47% days on TPN. Hence, it is interesting to note that the percentage of days on which the patient was fasting is lower, only 18.1%. This indicates that the TPN was associated with oral ingestion most of the time.
Several studies comparing EN with TPN reported that the use of nutritional therapy based on the GI tract is preferred as it is a more physiologic approach.20 Furthermore, TPN is associated with an increased risk of infections, especially in immunocompromised patients, which include patients submitted to HSCT.13,20 However, in Allogeneic HSCT, most patients progress to severe mucositis associated with thrombocytopenia, making EN less used in adult patients.
The percentage of patients with some degree of malnutrition on admission was only 3.1% and the percentage of overweight/obesity was 51.5%. Sucak et al., in a study with 71 patients, found a similar distribution in relation to the prevalence of malnutrition on admission (5.6%) but lower for overweight/obesity (39.5%).21
The patients showed a worsening in their nutritional status during hospitalization according to anthropometric (weight loss and decrease in BMI) and laboratory parameters (decrease in serum albumin levels). Body weight, as well as other parameters, has limitations for nutritional assessment during HSCT, especially in patients who use TPN as they can have increased body water, thereby masking the real weight loss.22
According to a survey of Japanese patients submitted to allogeneic HSCT and evaluated by bioelectrical impedance, more than half of the population (50.6%) had loss of muscle mass before transplantation. These data suggest that the nutritional status measured by weight and using BMI as a parameter, could mask a loss of muscle mass and the accumulation of fat mass.23
The nutritional status of patients during HSCT is not well documented in the literature. Few studies have evaluated the nutritional impact on adults of allogeneic HSCT.10,24 Park and Park evaluated the nutritional status before and after allogeneic HSCT and observed a negative impact on the nutritional status post-transplantation, but the relationship of nutritional status on the outcome of HSCT was not evaluated, for example, regarding the time of grafting or the appearance of GI and/or clinical complications.25 Sucak et al. observed a negative correlation between BMI of patients submitted to allogeneic HSCT and the development of symptoms and metabolic complications, such as mucositis, cardiotoxicity and hyperglycemia.21
Some studies indicate that the nutritional status of the patient before transplantation can affect the prognosis, and the extremes (malnutrition and obesity) are related to higher mortality and more complications associated with transplantation.7,10 In this scenario, specialized nutritional interventions may contribute to increased tolerance to chemotherapy and radiotherapy, contributing to the success of treatment.9,12,26
As in this study, Le Blanc, Ringdén and Remberger also found no correlation between nutritional status and time of neutrophil engraftment in patients submitted to allogeneic transplantation.27 Hadjibabae et al. however found a significant delay in engraftment of neutrophils and platelets in low weight patients submitted to allogeneic transplantation.22
Most studies on the relationship between nutritional status and post-transplant toxicity refer to adult patients submitted to autologous transplantation, which is a treatment modality relatively well tolerated in terms of toxicity.
Although the nutritional status does not present any major impact on immunological complications or tumor behavior, nutritional status may have an impact on the metabolism of chemotherapeutic agents. The decreased levels of plasma proteins and a reduced glomerular filtration rate may increase the free drug concentration and aggravate the cytotoxic effects in patients with low weight.21
Furthermore, the altered nutritional status, particularly malnutrition and obesity can have a negative impact on the risk of infection, which is considered to be the main cause of morbidity and mortality related to HSCT.8,21 Approximately 50% of patients remain with increased caloric and protein needs up to one year after HSCT.10
Hosley, Bauer and Gallagher found that patients classified as malnourished according to the Subjective Global Assessment (SGA) validated for cancer patients had a BMI within the normal range (23.8kg/m2).24 These results corroborate other studies showing that cancer patients classified as normal or overweight by BMI, could be classified as malnourished by the SGA, thus suggesting that the body fat of these individuals might be masking some degree of malnutrition not yet revealed by the weight.28
Although this study presents some limitations related to the sample size and the retrospective design, the results agree with previous studies, reiterating the impairment of nutritional status during the transplantation process.
ConclusionOur results suggest that patients submitted to allogeneic HSCT have a worsening in their nutritional status during hospitalization, mainly characterized by weight loss, high prevalence of GI symptoms and low dietary intake, probably due to the high toxicity related to this type of transplant and its complications. Thus it is important to analyze the factors involved in causing the nutritional deficits in order to implement early nutritional intervention in patients submitted to allogeneic HSCT.
Conflicts of interestThe authors declare no conflicts of interest.