There are several complications of hematopoietic stem cell transplantation. Without any doubt, most important of these is aGvHD that increases transplant-related mortality. The aim of this study is to investigate whether ST-2 and Reg3α levels measured at an early stage in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation can be individual biomarkers identifying future GvHD and predicting treatment response.
Materials and methodsFrom January 2019 to January 2021, 27 patients undergoing hematopoietic stem cell transplantation for primary immunodeficiency or hematopoietic diseases formed the study group. During their follow-up, the patients were classified into two groups as those developing and those not developing aGvHD. Nineteen healthy volunteers from a similar age group who needed their blood samples drawn for other reasons and who did not have any history of chronic disease, infection or medication use formed the control group. Blood samples of patients scheduled to have allogeneic HSCT were obtained before the administration of the preparative regimen, on Day +7 post-transplant and on the day of diagnosis if they developed aGvHD. Serum samples were stored at -20ºC until the day of processing. ST2 and Reg3α levels were measured using the ELISA method.
ResultsFor patients who developed aGvHD (n = 13), ST2 levels obtained before the transplantation, on Day +7 post-transplant and on the day of aGvHD diagnosis (in patients developing GvHD) were significantly higher compared to the healthy Control Group (p-value <0.05). As regards to the samples obtained on the same days, ST2 levels did not differ significantly among patients who developed and those who did not develop GvHD (n = 14; p-value >0.05). ST2 levels of samples obtained on the days that acute skin and gastrointestinal tract GvHD developed did not differ significantly between these two groups (p-value >0.05). Reg3α levels of the pre-transplant samples, on Day +7 after the transplantation and on the day of aGvHD diagnosis did not show any difference between any of the groups (p-value >0.05). As only two patients died after transplantation, thus correlation of ST2 and Reg3α levels with transplant-related mortality could not be proven.
ConclusionThe results of this study suggest that ST2 and Reg3α levels are neither diagnostic nor prognostic or predictive biomarkers of aGvHD, steroid resistance or transplant-related mortality in pediatric patients. This study can be regarded as a pilot study because of the small patient population; more research involving a larger patient population is required.
Hematopoietic stem cell transplantation (HSCT) is a treatment modality that allows the restructuring of defective and non-functional hematopoietic stem cells with healthy, normally proliferating and immunocompetent cells.1 HSCT is used for many diseases whether it be malignant or non-malignant. As in all other treatment modalities, HSCT can lead to many complications the most important being acute HSCT (aGvHD) as it increases transplant-related mortality. aGvHD can be seen in 35–45 % of fully matched transplants and in 60–80 % of partially matched transplants. Although the mortality rate is reported as 20 %, with the combination of clinical status and risk factors this might increase up to 80–90 %.2 Despite the use of several agents for treatment, aGvHD is still one of the most fearsome complications of HSCT3. For such an aggressive condition that is one of the most important causes of transplant-related mortality, there is an ongoing effort worldwide to find a prognostic biomarker that would predict its occurrence and to evaluate its treatment response. There are many studies conducted with adults, however a prognostic marker for pediatric aGvHD has not yet been found. In studies with adults, the two most frequently used biomarkers are ST2 (suppressor of tumorigenicity-2) and Reg3α (Regenerating islet-derived protein 3α). ST2 is the receptor of IL-33, a member of IL-1 family. This receptor has two forms: membrane binding and soluble. The soluble form prevents the interaction of IL-33 and membrane binding ST2 and it plays a role in the graft-versus-host disease (GvHD) pathophysiology. Reg3α is secreted from gastrointestinal epithelial cells and specifically damages Paneth cells.4,5 The studies that have so far been performed demonstrate an increase in blood levels of these two biomarkers before clinical and pathological signs of GvHD appear; they reflect six month-one year transplant-related mortality and play an important role in assessing treatment response on Day +28 of systemic steroid therapy6.
The aim of this study was to identify whether serum ST2 and Reg3α levels measured at an early stage can act as biomarkers that predict aGvHD which might develop after HSCT as well as predict treatment response.
Material and methodStudy designThe study group consisted of under 18-year-old patients who underwent HSCT at a University School of Medicine Pediatric Health and Diseases Bone Marrow Transplantation Unit from January 2019 to January 2021 with the diagnoses of primary immunodeficiency (PID) or hematological diseases. Patients were divided into two groups, those evolving with (Group 1) and not evolving with (Group 2) aGvHD during their follow up. The diagnosis of aGvHD was established by clinical signs and a biopsy and graded according to supporting histopathology. Nineteen healthy volunteers from the same age group who had their blood samples obtained for other reasons and who did not have a history of any chronic disease, infection or medication use served as the Control Group. This study was carried out at a tertiary academic center in concordance with international ethics standards and the World Health Organization Helsinki Declaration. Written approval was obtained from the University Faculty of Medicine Clinical Research Ethics Committee for this study.
Donors with a HLA match of 9/10 or above were defined as fully HLA matched and those with a match of 8/10 or below were defined as mismatched donors.
Sample collection and storageVenous blood samples were obtained from patients before starting the preparative regimen for allogeneic HSCT, on Day +7 post-transplant and on the day of aGvHD diagnosis if the disease developed. Serum was separated and the samples were kept at −20ºC until the day of analysis of ST2 and Reg3α levels.
Venous blood samples of age-matched healthy donors were obtained with serum being separated using the same method and kept at −20ºC until the day of processing.
ST-2 levels were measured with Cloud Clone Corp (ABD)/SEH820Hu 96 Tests – enzyme-linked immunosorbent assay kit for Interleukin-1 Receptor Like Protein (Organism Species: Homo Sapiens - Human). Moreover, Reg3α levels were measured with Cloud Clone Corp (ABD)/SEE675Hu 96 Tests - enzyme-linked immunosorbent assay kit for Regenerating Islet Derived Protein 3 Alpha (Organism Species: Homo Sapiens - Human) using the ELISA method.
Statistical analysisThe Statistical Package for Social Sciences (version 11.5) program was used for data analysis. For quantitative variables, descriptive tests are reported as means ± standard deviation and median (minimum-maximum) and for qualitative variables, percentages are used for numbers of patients. The differences between the qualitative variables that have two categories of quantitative variables were evaluated with the Student-t or the Mann–Whitney U test depending on whether they met normal distribution assumptions. The differences between the qualitative variables with more than two categories of quantitative variables were evaluated using the one-way ANOVA test if they met normal distribution assumptions. When the correlation between the two qualitative variables was analyzed, the chi-square and Fisher exact tests were used. Receiver operating characteristic (ROC) curve analysis was performed to find a method to use a gold standard quantitative variable instead of a qualitative variable; the Youden Index value was used to calculate cut-off values for qualitative variables. The level of statistical significance was set at 0.05.
ResultsCharacteristics of the patients and healthy control subjects included in the studyThe general characteristics of the 27 pediatric patients undergoing HSCT in the study group and the features of HSCT are shown in Table 1.
The general characteristics of the 27 patients in the study group and the features of hematopoietic stem cell transplantation.
WAS: Wiskott Aldrich syndrome; CGD: Chronic Granulomatous Disease HLH: Hemophagocytic lymphohistiocytosis; ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; DOCK-8: Dedicator of cytokinesis 8; IL10RA: Interleukin 10 receptor alpha subunit; SCID: severe combined immunodeficiency; CNI: Calcineurin inhibitor; MTX: Methotrexate; MMF: Mycophenolate mofetil; MSD: Matched sibling donor; MRD: Matched related donor; MMRD (Haploidentical): Mismatched related donor; MUD: Matched unrelated donor; PT-CY: post-transplant cyclophosphamide.
The most common complication that developed after HSCT was infection (14 patients), followed by aGvHD (13 patients - 48.1 %). Four patients were receiving intravenous antibiotic treatment for infection at the time of HSCT. While engraftment syndrome was observed in five cases, sinusoidal obstruction syndrome, one of the most serious complications after HSCT, was not observed in any case.
Of the Group 1 patients, eight (61.5 %) had skin, two (15.4 %) had gastrointestinal tract (GIS) and three (23.1 %) had both skin and GIS GvHD. Most of the patients (nine patients - 69.2 %) had Grade 1–2 aGvHD and only three (23.2 %) had Grade 3–4 aGvHD. Skin GvHD developed in a median of 27 days (range: 4–37 days) and GIS GvHD developed in a median of 75 days (range: 37–81 days).
All Group 1 patients received methylprednisolone (MPS) treatment on the day of GvHD diagnosis. Eight (61.5 %) of these patients did not respond to MPS treatment therefore one or more second line therapeutic agents were added to the regimen. The second line options were mycophenolate mofetil (MMF), cyclosporine, ruxolitinib, blinatumomab, tocilizumab and mesenchymal stem cell infusion.
Groups 1 and 2 did not differ significantly as regards to age, sex or diagnosis (p-value >0.05). Of the Group 1 patients, seven (53.8 %) had mismatched related (haploidentical) donors (MMRD). In Group 2, only one donor was MMRD. On comparing MMRD donors for Groups 1 and 2, donor type was found to be statistically significant for the development of GvHD (p-value = 0.013). The two groups did not differ significantly in terms of recipient-donor sex mismatch, recipient donor blood type incompatibility, infection status during transplantation, stem cell source, preparative regimen, GvHD prophylaxis and the given CD34 count (p-value >0.05). From the perspective of GvHD prophylaxis, seven of the eight cases (87.5 %) receiving post-transplant cyclophosphamide prophylaxis developed GvHD; however, this was not of statistical significance when compared with the other groups.
For Groups 1 and 2, complications, prognosis, follow-up times after HSCT were compared and no statistically significant result was found (p-value >0.05). Of the patients in the study group, two patients diagnosed with PID died after transplantation. The first of these cases was a ten-year-old girl with the diagnosis of DOCK8 deficiency. Transplantation was performed from her father who had HLA compatibility of 8/10 (MMRD) and used cyclophosphamide post-transplant. Four days after the transplant, she developed Grade 2 acute skin GvHD, 75 days later she developed Grade 2–3 acute GIS GvHD. GvHD was refractory to MPS treatment, therefore mesenchymal stem cell infusions, MMF, budesonide and tocilizumab were added. During her follow-up she died due to acute pancreatitis, sepsis and acute Grade 2–3 GIS GvHD. The second case was a six-month-old girl with the diagnosis of T- B+ NK- severe combined immunodeficiency (SCID). She received haploidentical HSCT with CD34+ cell selection from her father without conditioning. At the time of diagnosis and during transplantation she had cytomegalovirus (CMV) virus, CMV/Pneumocystis jirovecii pneumonia and CMV encephalitis; she developed Grade 1 skin GvHD 30 days after the transplantation. In addition to MPS, cyclosporine and MMF were used for treatment. The patient died due to acute respiratory failure associated with CMV pneumonia.
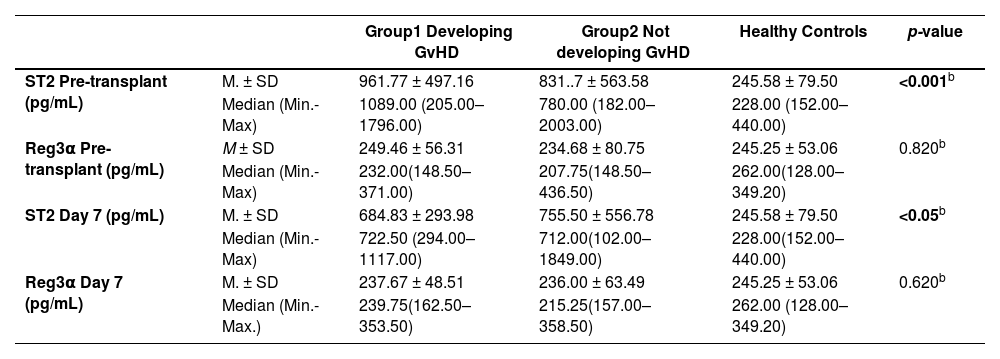
ST2 and Reg3α levels of patients undergoing HSCT and healthy control groupPre-transplant and Day 7+ post-transplant levels of ST2 in HSCT patients were found to be significantly higher than in healthy control subjects (p-value <0.05). The two groups did not differ significantly for the pre-transplant and Day +7 post-transplant levels of Reg3α (p-value = 1.00).
Table 2a shows ST2 and Reg3α levels of Group 1 and 2 patients and healthy control subjects.
Pre-transplant and 7 days post-transplant levels of ST2 and Reg3α of patients developing and not developing GvHD as well as healthy control subjects.
| Group1 Developing GvHD | Group2 Not developing GvHD | Healthy Controls | p-value | ||
|---|---|---|---|---|---|
| ST2 Pre-transplant (pg/mL) | M. ± SD | 961.77 ± 497.16 | 831..7 ± 563.58 | 245.58 ± 79.50 | <0.001b |
| Median (Min.-Max) | 1089.00 (205.00–1796.00) | 780.00 (182.00–2003.00) | 228.00 (152.00–440.00) | ||
| Reg3α Pre-transplant (pg/mL) | M ± SD | 249.46 ± 56.31 | 234.68 ± 80.75 | 245.25 ± 53.06 | 0.820b |
| Median (Min.-Max) | 232.00(148.50–371.00) | 207.75(148.50–436.50) | 262.00(128.00–349.20) | ||
| ST2 Day 7 (pg/mL) | M. ± SD | 684.83 ± 293.98 | 755.50 ± 556.78 | 245.58 ± 79.50 | <0.05b |
| Median (Min.-Max) | 722.50 (294.00–1117.00) | 712.00(102.00–1849.00) | 228.00(152.00–440.00) | ||
| Reg3α Day 7 (pg/mL) | M. ± SD | 237.67 ± 48.51 | 236.00 ± 63.49 | 245.25 ± 53.06 | 0.620b |
| Median (Min.-Max.) | 239.75(162.50–353.50) | 215.25(157.00–358.50) | 262.00 (128.00–349.20) |
a: Fisher-exact test;.
ST2 levels measured before the transplantation were found to be significantly higher in Groups 1 and 2 compared to healthy controls (p-value <0.001). Despite the fact that ST2 levels were numerically higher in Group 1, the difference between Groups 1 and 2 was not statistically significant (p-value = 0.684)
For the ST2 levels on Day +7 post-transplant, statistically significant differences were found between the study group and healthy controls (p-value <0.05). No significant difference was found between Groups 1 and 2 (p-value >0.05).
For pre-transplant and Day +7 post-transplant Reg3α levels, there was not any statistically significant difference between the study group and healthy control subjects (p-value >0.05).
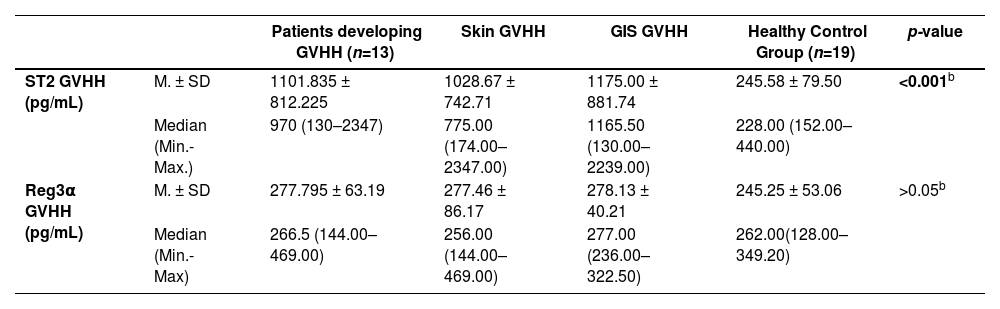
Table 2b presents ST2 and Reg3α levels measured on the day patients developed acute skin and/or acute GIS GvHD as well as in healthy controls.
ST2 and Reg3α of serum samples obtained on the day GvHD developed in aGvHD patients developing acute skin and/or acute GIS GvHD as well as healthy controls.
| Patients developing GVHH (n=13) | Skin GVHH | GIS GVHH | Healthy Control Group (n=19) | p-value | ||
|---|---|---|---|---|---|---|
| ST2 GVHH (pg/mL) | M. ± SD | 1101.835 ± 812.225 | 1028.67 ± 742.71 | 1175.00 ± 881.74 | 245.58 ± 79.50 | <0.001b |
| Median (Min.-Max.) | 970 (130–2347) | 775.00 (174.00–2347.00) | 1165.50 (130.00–2239.00) | 228.00 (152.00–440.00) | ||
| Reg3α GVHH (pg/mL) | M. ± SD | 277.795 ± 63.19 | 277.46 ± 86.17 | 278.13 ± 40.21 | 245.25 ± 53.06 | >0.05b |
| Median (Min.-Max) | 266.5 (144.00–469.00) | 256.00 (144.00–469.00) | 277.00 (236.00–322.50) | 262.00(128.00–349.20) |
a: Fisher-exact test;.
In Group 1 cases, ST2 levels obtained on the day of aGvHD diagnosis were significantly higher than those of healthy controls (p-value <0.001), however there was no significant difference between the groups with acute skin and those with acute GIS GvHD (p-value >0.05). In the comparisons of Reg3α levels obtained on the days that GvHD was diagnosed, no statistically significant differences were found between the three groups (p-value >0.05).
When the patients were compared in respect to the primary diagnoses that led to HSCT (PID and hematological diseases), ST2 levels measured pre-transplant and 7 days after the transplantation were not significantly different (p-value = 0.103 & p-value = 0.067) But, in the PID group other than acquired bone marrow failure, ST2 levels were higher compared to the other groups (p-value = 0.253 & p-value = 0.116).
For ST2 levels pre-transplant and 7 days after the transplantation, there was no statistically significant difference among patients who did not receive any preparative regimens, those who received myeloablative preparative regimens and those who received reduced intensity regimens (p-value >0.05).
No correlation of with age was identified in pre-transplant ST2 levels; in 27 patients who were transplanted and in 19 healthy controls, ST2 levels were observed to increase as age decreased.
In Group 1 patients who required second line treatment options due to steroid resistance, ROC curve analysis did not identify cut-off values for ST2 or Reg3α. Cut-off values could not be calculated for ST2 GIS GvHD and Reg3α GIS GvHD variables due to the limited number of patients who evolved with GIS GvHD. No significant differences were found for any of the quantitative variables (p-value >0.05). Data analysis did not allow evaluation of the prognosis or refractoriness to corticosteroids in this sample.
DiscussionThe hypothesis of this study was that ST2 and Reg3α levels could be elevated in the serum in pediatric patients who underwent HSCT before the appearance of aGvHD symptoms, that is, the levels could identify resistance to steroids in cases that will evolve with aGvHD and thus could predict transplant-related mortality.
Although the results of this study suggest that ST2 and Reg3α levels are not prognostic biomarkers in predicting aGvHD in the pediatric age group, the small number of patients may require this study to be considered as a pilot study.
The literature for the adult age group has many studies reporting ST2 and Reg3α as candidate biomarkers to predict the development of aGvHD and to evaluate treatment response. These studies have identified that ST2 and Reg3α can predict transplant-related mortality and steroid resistance. Blood levels of these two markers were shown to be elevated in patients developing aGvHD before the appearance of clinical symptoms.7-13 The common feature of such studies is that they were performed in adults and that malignant and non-malignant hematological diseases constituted the indications for allogeneic HSCT. The present study has significant merit in including a pediatric sample and patients with immune diseases where the expression of an immune marker such as ST2 might be impaired. This hypothesis can be adequately tested in a larger sample of SCID patients. There are few studies in the literature assessing biomarkers in pediatric patients and although less frequent than in adults, aGvHD causes considerable morbidity after allogeneic HSCT.
Some aspects of this study differ compared to research reported in the literature. The study group of this study consisted of patients who were solely in the pediatric age group. The indications for allogeneic HSCT were not limited to hematological diseases but also included PIDs. Moreover, serum samples of patients undergoing HSCT were obtained before the transplantation, on Day +7 after transplantation and on the day GvHD was diagnosed in patients.
In the literature on biomarker studies, a very limited number of studies analyze samples obtained before administering the preparative regimen. One, a multicenter study by Rowan et al., evaluated the prognostic significance of four biomarkers including ST2 and Reg3α. The study group included 170 under 10-year-old patients and 245 over 10-year olds. The most important difference of this study is the inclusion of the pediatric age group as well as the inclusion of pre-transplant serum samples. As in other studies, indications for allogeneic HSCT were limited to hematological diseases. Patient serum samples were obtained before the preparative regimen and on Days +7, +14 and +21 after transplantation. This study reported an increase in ST2 levels with age and a prediction of transplant-related mortality for under 10-year-old patients.14 In a study focusing on age, ST2 levels were shown to increase with age with higher levels being reported for men.15 In the current study, different to published findings, ST2 levels were found to increase as the age of the patients decreased. This difference might be explained by the limited number of patients in the study group.
In a study by Vander Lugt et al. involving 673 patients in 2000–2010, ST2 levels obtained 14 days after the transplantation were reported to predict transplant-related mortality and resistance to steroid therapy. Serum samples were collected starting on Day 0 up till Day +100 after the transplantation in patients who evolved with GvHD; serum samples were taken 48 h after initiating treatment. One of the most important findings of this study is that on Days 0, +14 and +21, ST2 levels were higher in the group receiving myeloablative preparative regimen compared to the group on a reduced intensity regimen.16 When the preparative regimen-ST2 correlation was evaluated in the current study, there was no significant difference between preparative regimens.
In the present study, ST2 levels were higher in Group 2. This might be explained by increases in ST2 due to other inflammatory conditions17. Other inflammatory states that cause elevated ST2 levels are inflammatory bowel diseases, chronic gastrointestinal inflammation, acute respiratory distress syndrome, asthma, allergies, non-allergic pulmonary diseases, heart failure, atopic dermatitis, sepsis and trauma.15,18-21 Having one or more of these inflammatory comorbid conditions in Group 2 might explain the high levels of ST2 seen in patients without GvHD compared to healthy controls.
In the present study, different to the literature,6,7,22 Group 1 patients who developed GIS GvHD did not have high levels of Reg3α or ST2 and the Reg3α levels were not significant in predicting steroid resistance in GvHD. This might be explained by the small number of number of patients with few cases evolving with acute GIS GvHD.
During the two years of the study, 27 patients underwent HSCT with two dying; both of these patients developed aGvHD. As the size of the study population was small and the number of patients who died was limited, the correlation of Reg3α and ST2 levels with transplant-related mortality could not be evaluated.
In a study by Balakrishnan et al. in 2016–2019 involving 210 patients with aGvHD, ST2 levels obtained 28 days after the transplantation predicted transplant-related mortality. The important features of this study were that the median patient age was 13.5 years and that serum samples were obtained before and after the administration of the preparative regimen and on Days +14 and +28 after the transplantation. Hematological diseases constituted the indications for allogeneic HSCT. In this study, ST2 levels before the administration of the preparative regimen tended to increase until Day +14.7 Different from the current study, ST2 levels obtained before the administration of the preparative regimen had a tendency to decrease until Day +7. In patients of Group 1, from Day +7 onwards, the levels increased until the day of GvHD diagnosis. We could not demonstrate that ST2 and Reg3α levels were specific to skin or GIS GvHD.
As the current study population was heterogeneous, one of the factors that could increase ST2 levels before the transplantation might have been the disease type. To this end, patients were categorized into four groups as SCID, PID other than SCID, and malignant and non-malignant hematological diseases. In the non-SCID PID group, ST2 levels were higher than the other groups, but this difference was not statistically significant. This difference might be explained by the presence of cases of immunodeficiency with immune dysregulation in the non-SCID immunodeficiency group.
There are certain limitations of this study. The COVID-19 pandemic affected this study as it affected every aspect of our lives. The drop in the number of donors from abroad and problems about donor safety during the COVID-19 pandemic resulted in a drop in the number of patients receiving transplants. As our study was prospective and longitudinal and as there was a limited study period, the number of patients enrolled was lower than anticipated. Having different results than the literature in the current study might be explained by the small number of patients.
In conclusion, this study is important as it questions the appropriateness of ST2 and Reg3α as individual biomarkers for predicting the development of aGvHD in pediatric patients undergoing HSCT. The results of this study suggest that ST2 and Reg3α levels are neither diagnostic nor prognostic or predictive biomarkers of aGvHD, steroid resistance or transplant-related mortality in the pediatric age group. This study can be regarded as a pilot study because of the small patient population; more research involving a larger patient population is required.