Acute myeloid leukaemia (AML) is considered a costly disease. Depending on the risk stratification, the patient may receive consolidation with cycles of intermediate doses of cytarabine, auto-HSCT or allo-HSCT according to availability in each service and the availability of a compatible donor. Literature data indicate that safety and effectiveness do not differ between consolidation therapy with intermediate-dose cytarabine or auto-HSCT, and so the cost can help physicians and health managers in their choice.
MethodThe cost of the second consolidation was compared in 18 to 60-year-old patients with de novo AML who were included in the International Consortium of Acute Myeloid Leukaemia (ICAML) protocol. Patients treated with auto-HSCT or intermediate doses of cytarabine (IDAC) were analysed during four years using the microcosting methodology.
ResultsThe mean costs for auto-HSCT and IDAC were BRL$ 34,900.95 (range: 23,611.36–41,229.59) and 15,231.64 (range: 6,546.36–23,253.53), respectively. The mean duration of in-hospital stay was 88.4 (93–133) and 94 (50–153) days, respectively. The mean cost of the four cycles of treatment was BRL$ 114.212,78 for auto-HSCT and BRL$ 121.980,93 for the chemotherapy group. Regardless of the type of treatment, the input that had the greatest economic impact was hospital admission, mainly due to infections.
ConclusionAuto-HSCT had a lower average cost per patient and hospitalization rate than chemotherapy.
For under 60-year-old patients with acute myeloid leukaemia (AML), most centres adopt the administration of anthracycline for three days and cytarabine for seven days (the so-called 3 + 7 regimen), which results in complete haematological remission (CR) in 60–80 % of cases1 and is a critical factor in increasing overall survival (OS). However, the vast majority of patients will relapse if they do not receive further therapy. As postinduction therapy, patients may receive chemotherapy with cytarabine, autologous haematopoietic stem cell transplantation (auto-HSCT) or allogenic haematopoietic stem cell transplantation (allo-HSCT), but the best postremission treatment strategy is still controversial. 1-5 According to clinical and genetic features at diagnosis, patients may be stratified as favourable, intermediate, or unfavourable, with the European LeukemiaNet Classification of 2017 being the most widely used criteria for this end.4 According to risk, donor availability, preferences/experience of the consulting physician, infrastructure and cost considerations, patients may receive allo-HSCT, chemotherapy or auto-HSCT as consolidation. In general, the treatment of choice for high-risk patients is allo-HSCT,4 whereas for low-risk patients, consolidation treatment may be based on cytarabine at doses of 2000–3000 mg per square metre of body-surface area (high-dose - HDAC) or 1000–1500 mg per square metre of body-surface area (intermediate-dose - IDAC).4–6 Different studies have suggested that treatment with IDAC and auto-HSCT are similarly effective as consolidation.1,6–13 For intermediate-risk patients, the best consolidation strategy is still controversial.4,11 Studies have shown that patients receiving auto-HSCT rather than high-dose chemotherapy have greater disease-free survival, lower relapse risk, lower treatment-related mortality due to lower toxicity, and a shorter duration of neutropenia but have a similar OS.3,7,12–15
The cost of acute myeloid leukaemia (AML) treatment is substantial and increasing.16 Pandya et al.16 analysed the healthcare costs of patients with AML undergoing various treatment approaches and showed that costs had increased in proportion to the general inflation in the USA between 1997 and 2018.16 Stein et al.17 calculated the expenses of 563 Medicare beneficiaries with AML for whom consolidation cycles were administered in an inpatient setting in approximately 65 % of the courses, with a mean duration of the inpatient stay of 8.6 days and a mean cost of US$28,843. Fewer than 10 % of the patients in this study received an HSCT, which was allogenic in more than 85 % of the cases. The mean duration of the inpatient stay for an HSCT was 34.3 days, and the mean cost was US$136,792.17 A study developed in the last decade in a public university hospital in Mexico estimated auto-HSCT costs per patient, including laboratory tests, medical procedures, chemotherapy drugs, other drugs, and hospitalization costs. The estimated total cost for an auto-HSCT procedure was US$12,504 with the most expensive components being drugs and laboratory tests. The study authors concluded that auto-HSCT is an affordable option for patients with haematological disease living in developing countries.18 However, the latter study included patients with different haematological malignancies, not only AML. To the best of our knowledge, there is no study comparing the costs of auto-HSCT with those of intermediate doses of cytarabine in the consolidation treatment of AML. Our hypothesis is that the use of auto-HSCT may reduce the duration of a patient's stay in the hospital and reduce the need for antifungal treatment, despite the costs related to HSCT harvesting. Indeed, Kurosawa et al.19 reported an incidence of invasive fungal infection (IFI) of 5.4 % in recipients of allo-HSCT, 0.4 % in auto-HSCT patients, and 0.8 % in patients receiving chemotherapy alone. These results suggest that auto-HSCT may be associated with fewer IFI episodes, consequently with shorter periods in the hospital and lower costs. Determining whether this hypothesis is valid may help health administrators and haematologists choose the most appropriate form of consolidation for AML patients.
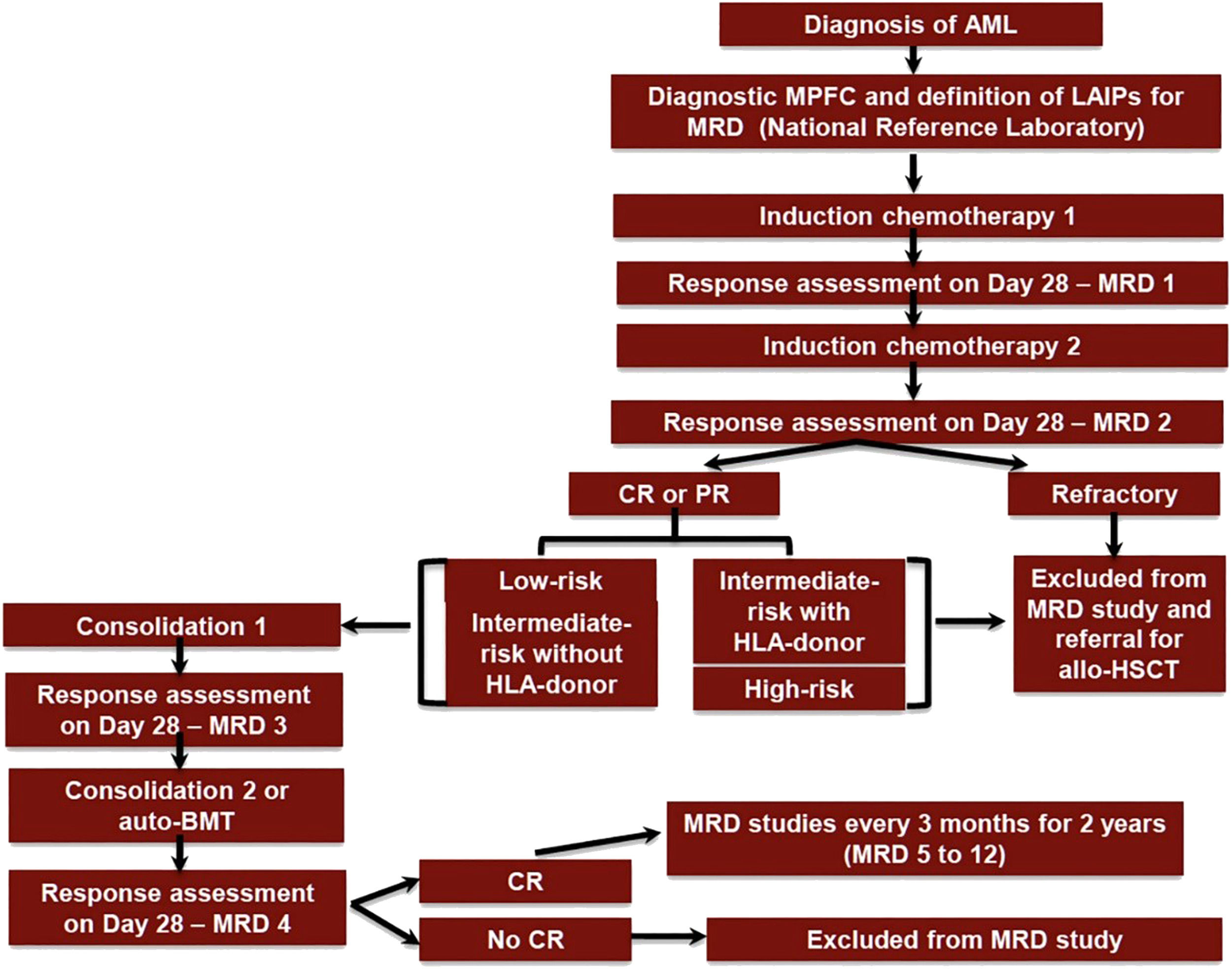
ObjectiveCompare the costs of the treatment of consolidation with auto-HSCT versus chemotherapy with IDAC for adult AML patients treated according to the International Consortium on Acute Leukaemias (ICAL) Acute Myeloid Leukaemia 2015 (ICAML2015) Study (ClinicalTrials.gov Identifier: NCT03023384) (Figure 1).20
ICAML2015 trial flow diagram. AML: acute myeloid leukaemia; Auto-BMT: autologous bone marrow transplant; CR: complete haematological remission; LAIPs: leukaemia-associated immunophenotype; MPFC: multiparameter flow cytometry; MRD: minimal residual disease; PR: partial response; HSCT: haematopoietic stem cell transplantation.
A retrospective analysis was performed of 18 to 60-year-old patients diagnosed with de novo AML (ICD-10 code: C92.0), who had intermediate risk genetic abnormalities according to the European LeukemiaNet 2017 classification.5 Only patients who did not have an HLA-matched donor were included. Patients were seen at Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto, University of São Paulo, from September 1, 2015 to September 1, 2019. Patients were included in the ICAML2015 study (ClinicalTrials.gov Identifier: NCT03023384) (Figure 1). Patients were treated equally in the first three cycles and grouped according to the type of treatment received in the last treatment cycle: auto-HSCT (Group A) or chemotherapy with cytarabine at a dose of 1 g/m2 bid for six days (IDAC - Group B). Data were obtained from the institution's cost records and through electronic medical records.
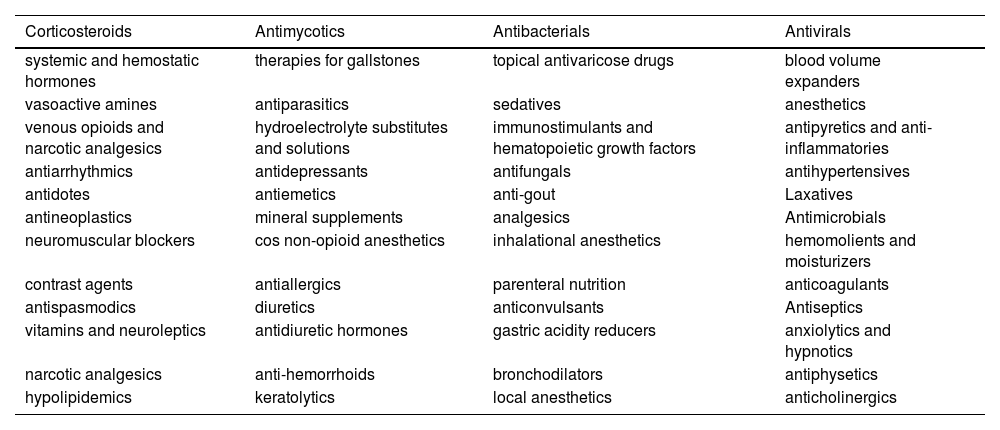
For cost analysis, information was initially collected about all resources and services consumed by patients, such as medicines according to the therapeutic class (Table 1).
Medicines consumed by patients according to the therapeutic class.
Additionally, all materials, laboratory analysis, units of blood transfusion components, apheresis, hospital admissions, and outpatient care were analysed, in the period that began on the date of diagnosis until hospital discharge after the fourth and last cycle. After collecting the quantitative data, the unit cost of inputs from the institution's perspective was used and updated by the value of the last year of the study, that is, 2019. The cost was analysed by treatment cycle, namely, first cycle of remission induction (RI-1), second cycle of induction (RI-2), first cycle of consolidation (CONS 1) and second cycle of consolidation (CONS 2), to have greater clarity of the economic impact per therapeutic cycle.
Unit costs for outpatient care and hospitalization were calculated in Brazilian Reals (BRL$) using the absorption costing methodology, which allocates all direct and indirect costs of the care provided. Input costs vary from year to year; consequently, to balance the cost of all inputs, they were adjusted to the mean cost of 2019 (cost basis). The mean cost of outpatient care covered medical consultations, dressings, paramedics, minor surgeries, and procedures.
If patients developed an IFI, the clinical manifestations, diagnostic criteria, aetiology, and treatment were recorded. The cases of IFI were classified according to the modified European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) criteria.21 Briefly, proven cases needed either a positive culture from a normally sterile fluid or tissue or visualization of fungi in tissue, whereas probable cases were defined on the basis of host factors, clinical features, and mycological criteria. Cases of possible IFI were excluded.
Pairwise comparisons between patient subgroups were performed using the Kruskal–Wallis test for continuous variables and by Pearson's chi-square test for categorical variables. All analyses were performed using R software package version v 3.5.1 (R Foundation for Statistical Computing; www.r-project.org) with a two-sided p-value <0.05 being considered statistically significant.
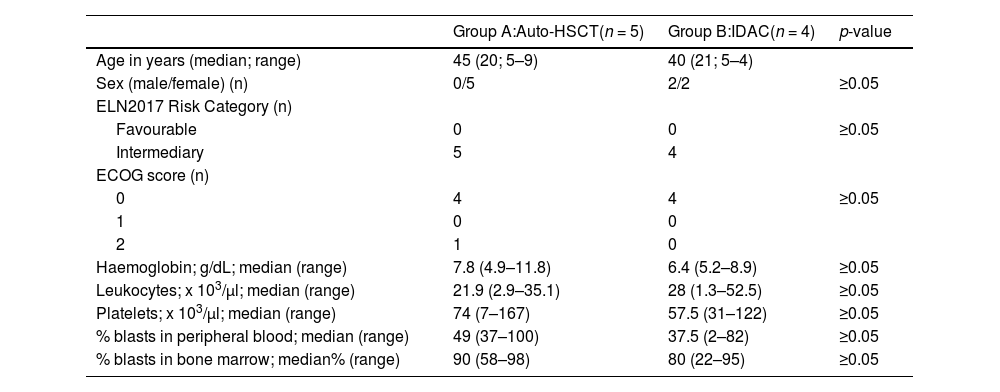
Results and discussionTable 2 shows the demographic and laboratory characteristics of the nine patients included in this analysis. The distribution of variables was similar between Groups A (auto-HSCT) and B (IDAC). Of note, according to the protocol, patients were eligible if the Eastern Cooperative Oncologic Group (ECOG) performance status was ≤ 2.
Demographic and laboratory characteristics.
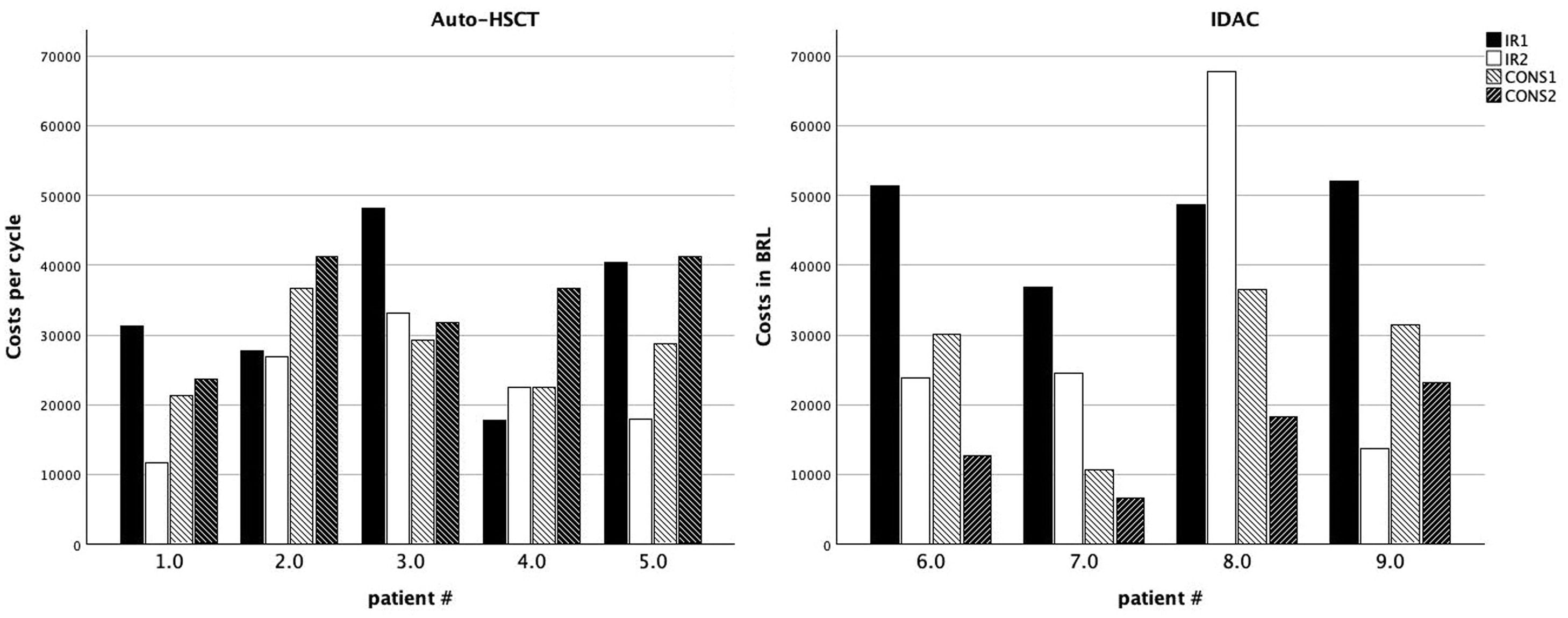
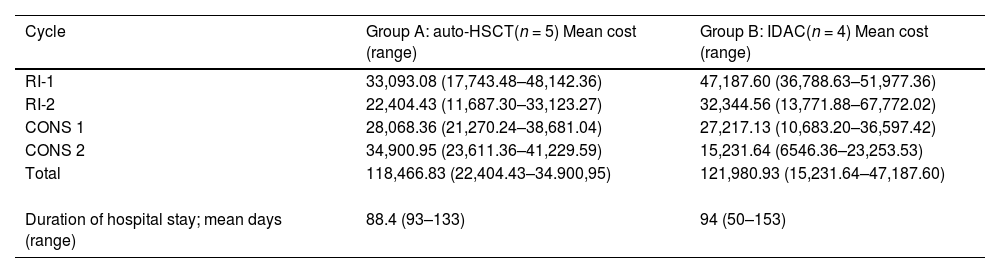
Table 3 shows the mean cost per cycle of treatment in Groups A and B. The aim of the present study was to evaluate the differences in CONS-2 with the results showing that the use of auto-HSCT was on average BRL$ 19,669.31 more expensive than IDAC. The mean duration of hospital stay during CONS-2 was 88.4 days (range: 93–133 days) for Group A and 94 days (range: 50–153 days) for Group B.
Mean cost per cycle of treatment per patient in Brazilian reals (BRL$).
RI-1: induction 1, RI-2: induction 2, CONS-1: consolidation 1, CONS-2: consolidation 2.
To better understand which factors influenced the cost of treatment and to analyse the variability between groups and during the different phases of treatment, the costs of RI-1, RI-2 and CONS-1 as well as the sum of the four cycles were also calculated. The mean total value per patient in Group A was BRL$ 118,466.83, whereas it was BRL$ 121,980.93 for those in Group B (Table 3).
Figure 2 shows the costs per patient per cycle. In five out of the nine patients, RI-1 was the costliest cycle of the treatment (for patient #5, the costs of RI-1 and CONS-2 were similar). The current results corroborate those of Pandya et al.,16 who reported that costs were higher for high-intensity induction chemotherapy (HIC-I), which was defined as inpatient high-dose cytarabine plus anthracycline use within three months of diagnosis, compared with those on high-intensity consolidation chemotherapy (HIC-C), defined as cytarabine plus anthracycline use during the two months following HIC. The authors reported costs of $198,657 and $73,428 for HIC-I and HIC-C, respectively.16 There is a relationship between the chemotherapy used in RI-1 and RI-2 and that of HIC-I and HIC-C, respectively. One possible explanation for the higher costs in RI-1 is the higher incidence of infectious complications, longer in-hospital stays, and the need for more frequent transfusions in patients with active disease, i.e., with higher infiltration of bone marrow and blood by leukaemic blasts.
Overall cost per treatment cycle for patients in Group A (auto-HSCT - A) and Group B (IDAC - B). Individual patients are presented on the X-axis. Bars represent the costs of induction cycle I (solid black), induction cycle II (solid white), consolidation I (white hatched) and consolidation II or auto-HSCT (black hatched).
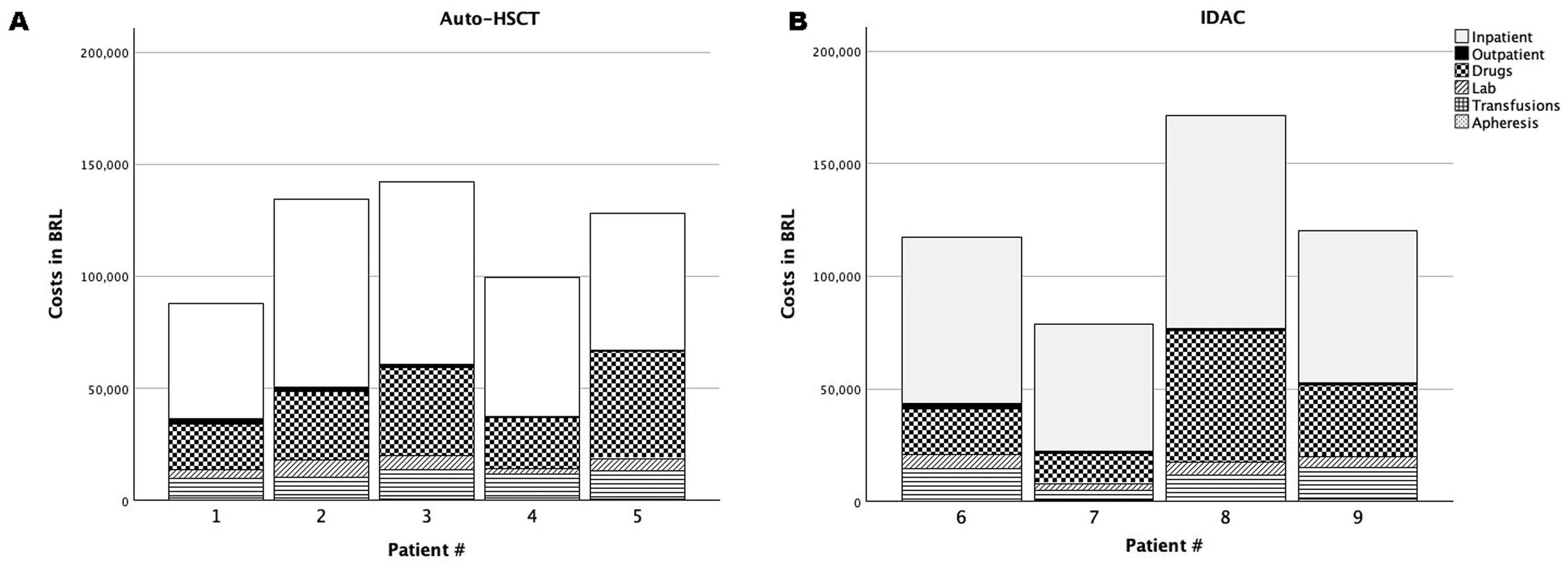
Figure 3 shows the costs per patient of inpatient and outpatient care including, drugs, laboratory analyses, units of blood transfusion components, and apheresis. The variables are grouped according to the treatment received in CONS-2 (auto-HSCT - A or IDAC - B). Regardless of the treatment cycle, hospital admissions (inpatient care) had the greatest economic impact representing 51.2 % of the total costs and corresponding to 47.7 % of the total costs in Group A and 59.9 % in Group B. Drugs had the second greatest economic impact with treatment of infections playing an important role in increasing the use of materials in general. For this reason, auto-HSCT or IDAC were analysed to see whether longer in-hospital stays and/or higher costs were related to the use of antibiotics and antifungal therapies. All patients in the study had febrile neutropenia that needed hospital admission. Three patients were treated for IFI episodes; all were considered probable according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group21 with the most frequently used antifungals being amphotericin B (deoxycholate) and voriconazole. All patients received prophylaxis with fluconazole. The frequency of IFI considering all patients was 11 % (4/36 episodes of febrile neutropenia); there was no significant difference between Groups A and B (10 % versus 12.5 %, respectively; p-value = 0.45).
Costs per patient of inpatient and outpatient care, drugs, laboratory analyses, units of blood transfusion components, and apheresis. Patients in group A (auto-HSCT) and group B (IDAC) are shown in the left (A) and right (B) graphs, respectively. Individual patients are presented on the X-axis. Bars represent costs with inpatient care (solid white), outpatient care (solid black), drugs (squares), laboratory analyses (white hatched), transfusions (horizontal lines) and apheresis (speckled).
A retrospective and multicentre study carried out in Brazil was conducted to characterize the epidemiology and burden of IFI in allo-HSCT and auto-HSCT recipients, as well as in patients with AML and myelodysplasia treated with intensive chemotherapy.22 In a one-year follow-up period, the incidence of IFI was 13 %, with aspergillosis being the most frequent agent, followed by candida and Fusarium. The incidence of IFI was higher in AML (26.1 %) patients, followed by ALL (16.7 %), allo-HCT (11.3 %), and auto-HCT (2.0 %). Antifungal prophylaxis was routinely administered to AML patients receiving chemotherapy or HSCT, and the choice of antifungal depended on the IFI risk assessment.22 The incidence detected in the present study was similar to that reported by Nucci et al.22 However, caution should be taken in interpreting the data of this study because of the small size of the cohort. On average, patients in Group B stayed 5.6 days longer in the hospital than those in Group A.
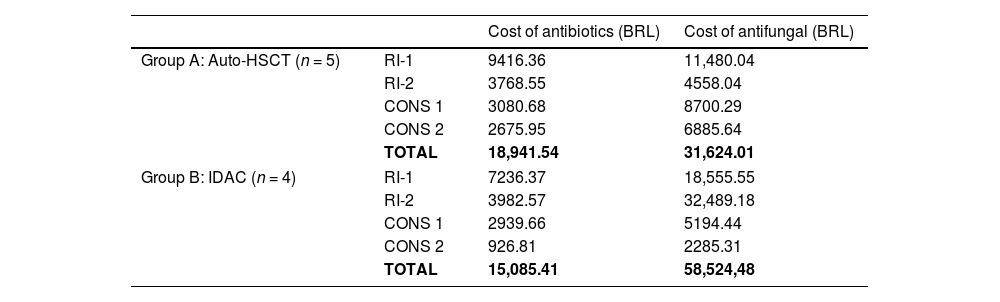
Table 4 shows the costs of antibiotics and antifungal therapies per cycle in each group. The first-class drugs resulted in similar costs between groups (Group A: BRL$ 18,941.54 versus Group B: BRL$ 15,085.41). On the other hand, consolidation with IDAC required considerably more antifungal treatment than auto-HSCT (Group B: BRL$ 31,624.01 versus Group B: BRL$ 58,524.48).
Costs of antimicrobial therapies per treatment cycle.
RI-1: induction 1, RI-2: induction 2, CONS 1: consolidation 1, CONS 2: consolidation 2, BRL: Brazilian $.
There is a paucity of studies on the intensive care costs of patients treated in low- and middle-income countries (LMICs). Tuon et al.24 performed a comparative cost-effectiveness analysis of the use of posaconazole versus fluconazole for antifungal treatment in patients with AML in Brazil. The total cost of using posaconazole was US$220,656.31, while that of fluconazole was US$83,875.00. These results showed that for patients with IFI who remained hospitalized for more than 12 days, the mean cost was US$850.85 per patient per day. The total money spent per private hospital on 100 patients for 100 days was US$342,318.00 for the posaconazole group and US$302,039.00 for the fluconazole group.23
In this study, hospitalization was a key factor in the cost of AML treatment regardless of the choice of consolidation, but it was not considered so in most cost studies in the literature.24 In 2018, Bell et al.25 published a retrospective analysis of the economic impact of treating elderly patients with AML in the USA. Ninety-two percent of the patients were hospitalized at least once, and in 39.2 % of the patients, admission to the intensive care unit (ICU) was needed. In the present study, no patient was admitted to the ICU. The mean total monthly cost per patient was US$25,243 during treatment, which was higher in the first year (US$27,756) than in the second year (US$12,953). Most of the total costs were medical (US$24,512), including hospitalizations (US$6548), outpatient visits (US$5021), supportive care (US$3640), and chemotherapy administration (US$2029). Therefore, hospitalization was responsible for 26.7 % of the costs.25
It should be pointed out that this study adopted a microcosting method, in contrast to gross costing studies that generally reflect reimbursement amounts or charges. Microcosting improves accuracy in cost estimation and reflects actual resource use and patient-specific costs, made possible by collecting detailed data on resources used and the unit costs of those resources. In this analysis, the unit cost of each resource through the institution's perspective was considered. This study provides an important view of the economic impact of the needs and choices of health services in the context of a middle-income country. The results of this study may help doctors and health managers in the proper direction of investments.
Study strengths and limitationsThe strength of this study is that it is based on cases that well represent the reality of AML treatment in middle-income countries and may help to improve the allocation of resources in the national health system. The main limitation is the number of patients. We included only patients who participated in the ICAML2015 trial, whose diagnosis and treatment were standardized and may be considered standard of care. We believe that our conclusions can be easily replicated in other centres.
ConclusionsThe results of this study show that the costs of consolidation with IDAC and those of consolidation with auto-HSCT are similar, but in the former, the costs related to antifungal therapy are higher. This is important in regions with higher frequencies of IFI.