to verify whether the review criteria for automated blood counts suggested by the International Consensus Group for Hematology Review of the International Society for Laboratory Hematology are suitable for the Hematology Laboratory of Hospital de Clinicas, Universidade Federal do Paraná.
Methodsinitially, the review criteria of the International Society for Laboratory Hematology were adapted due to limitations in the Institution's electronic hospital records and interfacing systems. The adapted review criteria were tested using 1977 samples. After this first assessment, an additional 180 inpatient samples were analyzed to evaluate the screening criteria of the review criteria in conjunction with positive smear findings established by the institution. The performance of the review criteria was verified by determining false positive, false negative, true positive and true negative rates, sensitivity, specificity, positive predictive value, negative predictive value, microscopic review rate and efficiency.
Resultsinitial analysis showed false negatives=6.73%, false positives=23.27%, microscopic review rate=46.03% and efficiency=70.0%. An evaluation of the screening criteria adapted from the review criteria together with the positive smear findings of the institution showed false negatives=15.5%, false positives=10.5%, microscopic review rate=37.3% and efficiency=73.8%. In both situations the safety limit (false negative <5%) recommended by the review criteria was exceeded.
Conclusionsthe review criteria adapted from the International Society for Laboratory Hematology are neither suitable nor safe for use in the hematology laboratory of the Hospital de Clinicas. This implies a need to develop and validate institution-specific review criteria in order to decrease false negative results to an acceptable and safe rate for patients.
In 2005, the International Society for Laboratory Hematology (ISLH) through the International Consensus Group for Hematology Reviews, founded by hematologist Berend Houwen, published a set of 41 rules applicable as criteria for the review of automated complete blood counts (CBCs) and leukocyte differential results of automated hematology analyzers, i.e., review criteria for automated complete blood counts (RC).1 These guidelines were formulated with the aims of reducing costs and the turnaround time of the results without sacrificing their quality, and justifying the performance and skills of the multiparametric hematology analyzers.2–4 Since then, the rules suggested by the ISLH2 have been considered the international standard to indicate situations requiring a blood smear review (BSR). They take into account the age and gender of patients, whether the request for CBC is the initial or a subsequent one to monitor CBCs, or whether there are significant differences between the current results, and previously validated and released results.2,4 In practice, they are based on the set of screening thresholds for the results given by the analyzers and in the presence or absence of suspect flags. The aim is to distinguish samples with a high probability of containing relevant morphological alterations for the diagnosis and treatment of patients. When the CBC results do not meet the screening criteria, there are recommended procedures to follow, specifically to prepare an adequate peripheral blood smear for microscopic analysis.2
Hospital de Clínicas of the Universidade Federal do Paraná (HC-UFPR) is a general Class IV hospital according to the hospital classification system of Brazil's publically funded healthcare system (SUS); it is the largest provider of government healthcare services in the State of Paraná with 510 beds. Moderately to highly complex procedures are carried out in 59 departments. Approximately 61,000 consultations are made per month. The clinical hematology laboratory is located in the Diagnosis Support Service and contains two types of hematology analyzers: the Sysmex XE-2100D and XT-2000i (Sysmex Corporation, Kobe, Japan). Approximately 500 samples are sent for CBCs daily. Prior to the development of the RC, 100% of CBCs were analyzed microscopically, which led to delays in the release of the results even when performed by experienced professionals.
According to Bain5 because BSRs and manual differential leukocyte counts (MDLCs) are laborious and expensive, they should be based on the RC. Thus, all hematology laboratories must be encouraged to establish locally valid protocols indicating when a BSR and MDLC should be performed. The guidelines suggested by the ISLH can be the starting point as long as they are interpreted in consideration of the experience of the laboratory staff, sophistication of the hematology analyzers and the laboratory's electronic records system, and incidences of abnormalities and variations in reference values of the population being tested.6,7 Thus, this study evaluated the implementation of the RC suggested by the ISLH in the HC-UFPR Hematology Laboratory in order to determine automated thresholds such that microscopic analyses are performed only under special circumstances. In addition, the study aimed to define whether such guidelines could be tailored to the population served or whether there is a need to establish and evaluate specific RC for this Institution.
MethodsStudy site and sample preparationThe investigation was conducted in the Hematology Laboratory of HC-UFPR after approval by the local Ethics Committee. The samples were obtained in two stages. First, for five consecutive days, all laboratory samples were collected after the release of the results into the electronic hospital records system. A total of 1977 whole-blood samples in ethylenediaminetetraacetic acid (EDTA)-K2 (1.8mg/mL) were analyzed within 3h of collection. Of these, 1573 and 404 were analyzed using the XE-2100D and XT-2000i hematology analyzers, respectively. Furthermore, to evaluate the screening criteria adapted from the ISLH together with positive smear findings (PSFs) of the HC-UFPR, an additional 180 inpatient samples were collected randomly and analyzed using the XT-2000i device; these samples were more likely to have PSFs because they also had abnormal CBC results. The PSFs elaborated by the HC-UFPR were intended to ensure clinically significant abnormalities were not omitted from the results, thereby establishing a minimum threshold of information that should be reported in the CBC results according to local consensus. All numerical data and information from suspect flags and blood smear findings were recorded. Approximately 70% of the samples tested were from outpatients, many of whom were having their first blood count. The other 30% were from inpatients from various hospital units (e.g., hematology, chemotherapy, infectious diseases, intensive care units, emergency care), many of whom had their blood counts monitored daily.
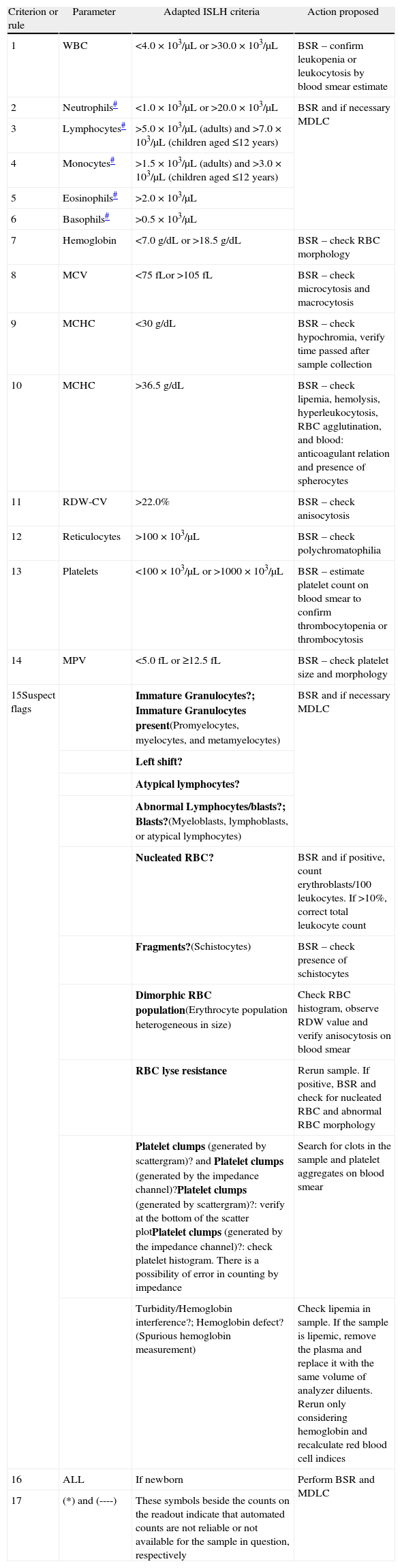
Adaptation of the review criteria of the International Society for Laboratory Hematology according to local requirementsIn order to determine whether the performance of these RC met local requirements or indicated the need to develop specific RC, the screening criteria and PSFs suggested by the ISLH were initially evaluated. However, changes were made to tailor the ISLH screening criteria to the hematology analyzers used in this study and particularly to adapt them to the electronic hospital records system. The main adaptations were associated with Delta Check rules, which were not possible to implement because of limitations of the institution's electronic hospital records and interfacing systems. The adapted ISLH screening criteria concerning the possibility of local implementation are shown in Table 1. Table 2 shows the PSFs recommended by the ISLH.2 PSFs that differ from those recommended by the ISLH were also created for the HC-UFPR (Table 3) in an attempt to meet local requirements.
Adapted International Society of Laboratory Hematology review criteria for automated complete blood counts.
| Criterion or rule | Parameter | Adapted ISLH criteria | Action proposed |
| 1 | WBC | <4.0×103/μL or >30.0×103/μL | BSR – confirm leukopenia or leukocytosis by blood smear estimate |
| 2 | Neutrophils# | <1.0×103/μL or >20.0×103/μL | BSR and if necessary MDLC |
| 3 | Lymphocytes# | >5.0×103/μL (adults) and >7.0×103/μL (children aged ≤12 years) | |
| 4 | Monocytes# | >1.5×103/μL (adults) and >3.0×103/μL (children aged ≤12 years) | |
| 5 | Eosinophils# | >2.0×103/μL | |
| 6 | Basophils# | >0.5×103/μL | |
| 7 | Hemoglobin | <7.0g/dL or >18.5g/dL | BSR – check RBC morphology |
| 8 | MCV | <75fLor >105fL | BSR – check microcytosis and macrocytosis |
| 9 | MCHC | <30g/dL | BSR – check hypochromia, verify time passed after sample collection |
| 10 | MCHC | >36.5g/dL | BSR – check lipemia, hemolysis, hyperleukocytosis, RBC agglutination, and blood: anticoagulant relation and presence of spherocytes |
| 11 | RDW-CV | >22.0% | BSR – check anisocytosis |
| 12 | Reticulocytes | >100×103/μL | BSR – check polychromatophilia |
| 13 | Platelets | <100×103/μL or >1000×103/μL | BSR – estimate platelet count on blood smear to confirm thrombocytopenia or thrombocytosis |
| 14 | MPV | <5.0fL or ≥12.5fL | BSR – check platelet size and morphology |
| 15Suspect flags | Immature Granulocytes?; Immature Granulocytes present(Promyelocytes, myelocytes, and metamyelocytes) | BSR and if necessary MDLC | |
| Left shift? | |||
| Atypical lymphocytes? | |||
| Abnormal Lymphocytes/blasts?; Blasts?(Myeloblasts, lymphoblasts, or atypical lymphocytes) | |||
| Nucleated RBC? | BSR and if positive, count erythroblasts/100 leukocytes. If >10%, correct total leukocyte count | ||
| Fragments?(Schistocytes) | BSR – check presence of schistocytes | ||
| Dimorphic RBC population(Erythrocyte population heterogeneous in size) | Check RBC histogram, observe RDW value and verify anisocytosis on blood smear | ||
| RBC lyse resistance | Rerun sample. If positive, BSR and check for nucleated RBC and abnormal RBC morphology | ||
| Platelet clumps (generated by scattergram)? and Platelet clumps (generated by the impedance channel)?Platelet clumps (generated by scattergram)?: verify at the bottom of the scatter plotPlatelet clumps (generated by the impedance channel)?: check platelet histogram. There is a possibility of error in counting by impedance | Search for clots in the sample and platelet aggregates on blood smear | ||
| Turbidity/Hemoglobin interference?; Hemoglobin defect?(Spurious hemoglobin measurement) | Check lipemia in sample. If the sample is lipemic, remove the plasma and replace it with the same volume of analyzer diluents. Rerun only considering hemoglobin and recalculate red blood cell indices | ||
| 16 | ALL | If newborn | Perform BSR and MDLC |
| 17 | (*) and (----) | These symbols beside the counts on the readout indicate that automated counts are not reliable or not available for the sample in question, respectively |
MCV: mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; RDW-CV: red blood cell distribution width coefficient of variation; RBC: red blood cells; WBC: white blood cells; BSR: blood smear review; MDLC: manual differential leukocyte count.
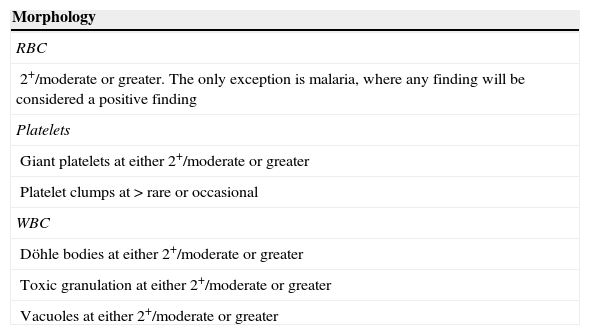
Criteria for a positive smear recommended by the International Society of Laboratory Hematology.
| Morphology |
| RBC |
| 2+/moderate or greater. The only exception is malaria, where any finding will be considered a positive finding |
| Platelets |
| Giant platelets at either 2+/moderate or greater |
| Platelet clumps at > rare or occasional |
| WBC |
| Döhle bodies at either 2+/moderate or greater |
| Toxic granulation at either 2+/moderate or greater |
| Vacuoles at either 2+/moderate or greater |
| Abnormal cell types |
| Blasts≥1% |
| Myelocytes/promyelocytes≥1% |
| Metamyelocytes>2% |
| Atypical lymphocytes>5% |
| Nucleated RBC≥1% |
| Plasma cells≥1% |
The International Society of Laboratory Hematology recommends that the use of band cell counts and left shift suspect flag (Left Shift?) should be in accordance to laboratory standard operating procedures. Thus, the Left Shift? Suspect flag was used as a screening criterion in this study and the band count was considered a positive smear finding when it was>8%.
RBC: red blood cells; WBC: white blood cells; SOP: standard operating procedure.
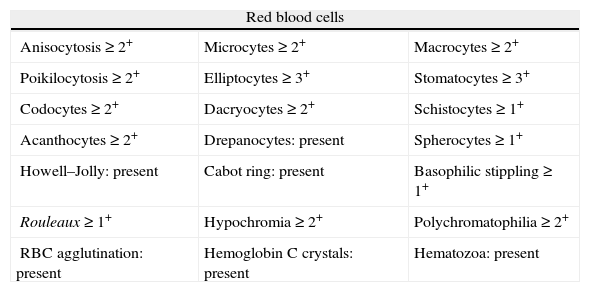
Criteria for a positive smear recommended by the Hospital de Clínicas da Universidade Federal do Paraná.
| Red blood cells | ||
| Anisocytosis≥2+ | Microcytes≥2+ | Macrocytes≥2+ |
| Poikilocytosis≥2+ | Elliptocytes≥3+ | Stomatocytes≥3+ |
| Codocytes≥2+ | Dacryocytes≥2+ | Schistocytes≥1+ |
| Acanthocytes≥2+ | Drepanocytes: present | Spherocytes≥1+ |
| Howell–Jolly: present | Cabot ring: present | Basophilic stippling≥1+ |
| Rouleaux≥1+ | Hypochromia≥2+ | Polychromatophilia≥2+ |
| RBC agglutination: present | Hemoglobin C crystals: present | Hematozoa: present |
| White blood cells | ||
| Döhle bodies≥1+ | Toxic granulation≥1+ | Cytoplasmic vacuoles≥1+ |
| Polylobocytes≥1+ | Hyposegmented neutrophils:≥2+ | Neutrophil hypo/degranulation: present |
| Auer rod: present | Pseudo-Pelger-Huët: present | Dysplastic cells: present |
| Platelets | ||
| Giant platelets≥1+ | Microplatelets≥2+ | Platelet aggregates: present |
| Platelet anisocytosis≥3+ | Degranulated platelets: present | Gray platelets: present |
| Abnormal cell types | ||
| Blasts≥1% | Promyelocytes≥1% | Myelocytes≥1% |
| Metamyelocytes≥2% | Bands>8% | NRBC≥1/100 leukocytes |
| Plasma cells≥1% | Prolymphocytes≥1% | Atypical lymphocytes≥5% |
RBC: red blood cells; NRBC: nucleated red blood cells.
The criteria followed to select samples for review were compared with the findings of the peripheral BSR. A sample was classified as true positive (TP) if it was positive for a particular screening criterion (Table 1) and the microscopic analysis produced some PSF (Tables 2 and 3). Meanwhile, a sample was classified as false positive (FP) if it was positive for a particular screening criterion with no PSF in the microscopic analysis. A sample was classified as false negative (FN) if it was negative for all screening criteria and contained some PSF in microscopic analysis. Finally, a sample was classified as true negative (TN) if it was negative for all screening criteria and the BSR did not show any PSFs.2,8
ConsiderationsGood laboratory practices and procedures for quality assurance and quality control in hematology were followed to ensure good performance. All settings and configurations of the hematology analyzers followed the manufacturers’ recommendations. For all samples, the BSR and MDLC were performed according to an adapted version of the H20-A2 Clinical and Laboratory Standards Institute (CSLI),9 because each BSR and each MDLC was carried out by a single observer. In most cases, 100 leukocytes were counted. In some cases with leukocyte counts <0.03×103/μL, leukocytes were not observed and only a BSR could be performed. In other cases with leukocyte counts above 50.0×103/μL, the MDLC reached 200 leukocytes. Furthermore, in all samples, the BSR was performed to identify any qualitative or quantitative changes in red and white blood cells as well as platelets. Guidelines were formulated to standardize the quantification of morphologic alterations and which terms are used to report changes in CBC results. These guidelines were codified as standard operating procedures (SOP).
Statistical analysisThe sensitivity, specificity, positive predictive value, negative predictive value, microscopic review rate, and efficiency of the RC adapted from the ISLH were calculated as follows: sensitivity (%)=TP/(TP+FN)×100; specificity (%)=TN/(TN+FP)×100; positive predictive value (%)=TP/(TP+FP)×100; negative predictive value (%)=TN/(TN+FN)×100; microscopic review rate (%)=(TP+FP)/(TP+FP+FN+TN)×100; and efficiency (%)=(TP+TN)/(TP+FP+FN+TN)×100.8
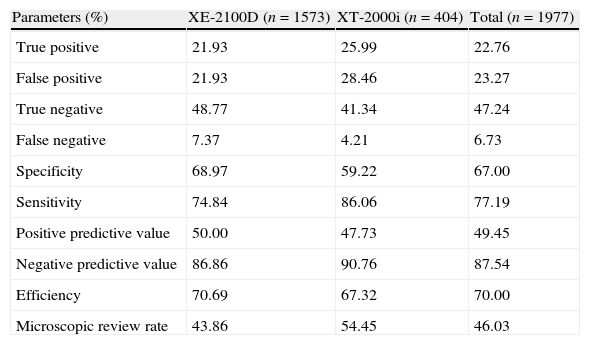
ResultsOut of the 1977 samples used in the initial investigation of the adapted RC, 583 (29.49%) were positive and 1394 (70.51%) were negative for relevant microscopic results, i.e., for PSFs. Most samples failed to meet some screening criteria; usually from one to four criteria but up to 15. The results obtained for the validation of the screening criteria tailored from the ISLH criteria, considering the positive smear criteria in Table 2, are shown in Table 4.
Truth table of the adapted International Society of Laboratory Hematology criteria.
| Parameters (%) | XE-2100D (n=1573) | XT-2000i (n=404) | Total (n=1977) |
| True positive | 21.93 | 25.99 | 22.76 |
| False positive | 21.93 | 28.46 | 23.27 |
| True negative | 48.77 | 41.34 | 47.24 |
| False negative | 7.37 | 4.21 | 6.73 |
| Specificity | 68.97 | 59.22 | 67.00 |
| Sensitivity | 74.84 | 86.06 | 77.19 |
| Positive predictive value | 50.00 | 47.73 | 49.45 |
| Negative predictive value | 86.86 | 90.76 | 87.54 |
| Efficiency | 70.69 | 67.32 | 70.00 |
| Microscopic review rate | 43.86 | 54.45 | 46.03 |
Note: the adapted ISLH screening criteria were tested considering the ISLH criteria for a positive smear as shown in Table 2.
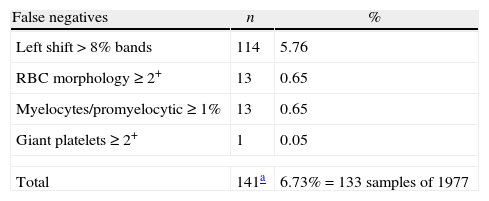
The microscopic review rate during the initial investigation (Table 4) was quite high (46.03%) compared to the 30% recommended by the American College of Pathologists.11 The main rules producing FP results in the initial assessment were as follows: leukocytes <4.0×103/μL (16.7% of total FPs), platelets <100×103/μL (13.3% of total FPs), and suspect flags (30% of total FPs). In the initial assessment of the RC adapted from the ISLH criteria, there were 133 FN samples (Table 5) including 114 with nuclear shift to the left in >8% of bands, 13 myelocytes/promylocytes≥1%, 13 alterations in red blood cells, and one alteration in platelets.
Incidence of false negatives: the adapted International Society of Laboratory Hematology criteria considering the International Society of Laboratory Hematology criteria for a positive smear.
| False negatives | n | % |
| Left shift>8% bands | 114 | 5.76 |
| RBC morphology≥2+ | 13 | 0.65 |
| Myelocytes/promyelocytic≥1% | 13 | 0.65 |
| Giant platelets≥2+ | 1 | 0.05 |
| Total | 141a | 6.73%=133 samples of 1977 |
RBC: Red blood cell.
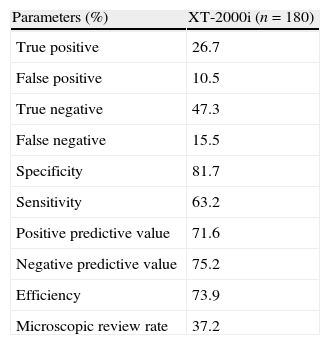
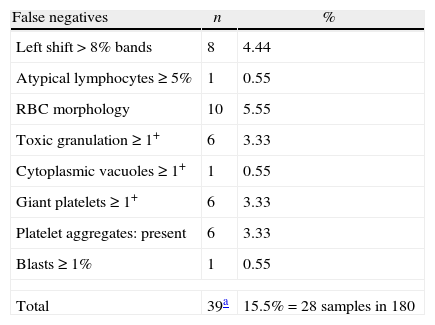
The evaluation results for the screening criteria adapted from the ISLH in conjunction with the PSFs of the HC-UFPR are shown in Tables 6 and 7. From the 180 samples analyzed, 76 contained PSFs. Using PSFs specific for the HC-UFPR, which were tailored to meet the requirements of the authors’ Institution, caused the screening criteria adapted from the ISLH to be incapable of filtering samples with relevant morphological alterations (FN=15.5%), including one case with blasts present from a patient with acute leukemia.
Truth table of the adapted International Society of Laboratory Hematology criteria considering the Hospital de Clínicas da Universidade Federal do Paraná criteria for a positive smear.
| Parameters (%) | XT-2000i (n=180) |
| True positive | 26.7 |
| False positive | 10.5 |
| True negative | 47.3 |
| False negative | 15.5 |
| Specificity | 81.7 |
| Sensitivity | 63.2 |
| Positive predictive value | 71.6 |
| Negative predictive value | 75.2 |
| Efficiency | 73.9 |
| Microscopic review rate | 37.2 |
Incidence of false negatives: the adapted International Society of Laboratory Hematology screening criteria considering the Hospital de Clínicas da Universidade Federal do Paraná criteria for a positive smear.
| False negatives | n | % |
| Left shift>8% bands | 8 | 4.44 |
| Atypical lymphocytes≥5% | 1 | 0.55 |
| RBC morphology | 10 | 5.55 |
| Toxic granulation≥1+ | 6 | 3.33 |
| Cytoplasmic vacuoles≥1+ | 1 | 0.55 |
| Giant platelets≥1+ | 6 | 3.33 |
| Platelet aggregates: present | 6 | 3.33 |
| Blasts≥1% | 1 | 0.55 |
| Total | 39a | 15.5%=28 samples in 180 |
RBC: Red blood cell.
In both analyses, the percentage of FN results exceeded the reliability threshold of 5% established by the ISLH, causing cases with serious illnesses to be overlooked.
DiscussionDespite the extensive capabilities of the latest-generation multiparametric hematology analyzers, microscopic review of blood smears still plays an important role in hematology laboratories. The use of RC, which would allow the release of automated counts without a BSR, is neither a widespread nor standardized procedure. In practice, many laboratories simply adopt published criteria or alter some criteria without empirical evidence.12
Establishing and assessing a specific RC for a particular laboratory means that the ISLH screening and positive smear criteria must first be validated, especially because these criteria are international standards. Considering the actual demands and capabilities of each laboratory, this initial validation clarifies whether such criteria require optimization or not.
In this study, after the initial evaluation of the RC adapted from the ISLH, 6.73% and 23.27% of FN and FP results were verified, respectively. Moreover, the microscopic review rate was 46.03% and efficiency was 70.0%. The microscopic review rate with the XT-2000i analyzer (54.45%) was higher than that with the XE-2100D (43.86%). This is because the former analyzer was used by staff members on duty, who only attend inpatients and emergency patients, who evidently have CBC results that differ substantially from those of outpatients.
The negative predictive value determined in the initial evaluation using the screening criteria and positive smear criteria showed that in 87.54% of the times in which the screening criteria adapted from ISLH did not indicate the need for BSR, the sample analyzed really did not contain any PSFs. The observed sensitivity was 77.19%, indicating that out of 583 samples, 450 with PSFs were correctly screened by applying the RC adapted from the ISLH. As mentioned above, the percentage of microscopic reviews (46.03%) greatly exceeded the 30% recommended by the American College of Pathologists. The fact that 30% of the FP results were of samples with suspect flags with a nonexistent microscopic counterpart indicated that the hematology analyzers used were guilty of over-flagging, i.e., they gave more warnings than necessary. As the sensitivity of the suspect flags had been adjusted by technicians of the manufacturer, we suggest that each laboratory should evaluate the efficiency of each suspect flag from the analyzers, thereby making proper adjustments to the sensitivity of the hematology analyzer or defining whether a suspect flag is actually useful as a screening criterion.
A total of 13.3% of samples had a platelet count <100×103/μL in the initial evaluation of the RC adapted from the ISLH; this is relatively high considering the sampling was representative of the local reality. Therefore, the microscope estimate of the platelet count should always be performed on samples with this profile in order to verify how well it complies with automated counting and to search for platelet aggregates and giant platelets, which are factors that produce underestimates.13
Moreover, the screening criteria adapted from the ISLH were tested according to the PSFs of the HC-UFPR; 15.5% and 10.5% of FNs and FPs were verified, respectively. In addition, the microscopic review rate was 37.3%, and the efficiency was 73.8%. One FN sample contained blasts. It is unacceptable to fail to detect cases of undiagnosed hematological malignancies; therefore, each institution should evaluate the need to perform BSRs in all patients in the hematology unit even at the expense of an increased microscopic review rate.
Despite some studies suggesting poor clinical practice when counting bands,14–16 in this study, a band count>8% was chosen as the positive smear criterion, particularly because a substantial proportion of doctors in Brazil posit there are associations of a nuclear shift to the left of neutrophils with infectious and inflammatory conditions. The FN analysis in the present study revealed a high occurrence of a nuclear shift to the left>8% bands. Nevertheless, the technical limitations of MDLC must be taken into account when interpreting these results, especially results regarding band counts and variations occurring as a result of age, gender, and conditions such as pregnancy and physical exercise.15,17
The greatest amendment made to the RC of the ISLH was regarding the Delta check rules. These rules recognize morphological abnormalities detected and validated in previous analyses as well as discrepancies between the results of the current analysis and previous results from up to five days earlier. The Delta check limit threshold for a particular parameter is the amount by which a result can differ from a previous one; this difference can be expressed as the percentage difference or the absolute value in the unit of the hematological parameter in question. Delta limits should be established by each laboratory taking into account the physiopathological aspects and technical characteristics of the hematology analyzers used.
Although the Delta check rules play important roles in the efficiency and reliability of the CBC results directly released without a BSR, many clinical laboratories are incapable of implementing them in their electronic records or interfacing systems because of high software development costs. Even in developed countries, some laboratories are still incapable of modifying their electronic record systems to incorporate such regulations.18
Another equally important factor is how unfamiliar laboratory professionals are with these rules, which make their dissemination and implementation difficult. The ISLH does not suggest specific Delta check limits, leaving them to the discretion of the laboratory. This could lead to small differences in the efficiency of the ISLH criteria when applied by different laboratories using different Delta limits since the International Consensus Group for Hematology Reviews only suggests specific actions for situations in which the Delta limits individually established by the laboratories are exceeded.
ConclusionsNeither assessment carried out in this study proved to be reliable, because the FN rates exceeded 5%. Moreover, the microscopic review rates were high, which may pose problems when attempting to decrease the turnaround time of results and when laboratories have an insufficient number of experienced professionals to perform the BSR. Therefore, in conclusion, new criteria should be developed and evaluated taking into account local peculiarities, requirements, and opportunities while aiming not to overlook samples with PSFs. To this end, additional studies are being carried out by the authors with the aim of developing a method to help establish and evaluate the efficiency of criteria for reviewing automated CBC results.
Conflict of interestThe authors declare no conflicts of interest.