Despite an increase in the rate of successful live donor renal transplantation done annually, the number of potential recipients with acceptable donors is relegated to the ever-expanding cadaver-donor waiting list due to sensitization to human leukocyte antigen (HLA) antibodies. If not sufficiently suppressed, these preformed HLA antibodies can trigger antimicrobial resistance (AMR) and early graft loss. To ameliorate this situation, various desensitization treatments are administered to provide a survival benefit to highly sensitized patients.
MethodOne hundred and six patients in the time frame of January 2017 to March 2019 were included in the study group. The desensitization protocol included therapeutic plasma exchange and administration of low-dose intravenous immunoglobulin (100 mg/kg per therapeutic plasma exchange (TPE) session) to highly sensitized patients (treatment group) who subsequently underwent renal transplantation after negative pre-transplant Centers for Disease Control and Prevention Luminex crossmatch (CDC/LumXM). We compared graft survival rates between the group undergoing desensitization (treatment group) and matched control group of patients that underwent HLA-compatible transplantation.
ResultsIn the treatment group, Kaplan-Meier analysis estimates an average rate of patient graft survival of 95.2% at 3 years post-transplant, as compared with the rate of 86.9% in the same time frame for the control-matched group (p < 0.05 for both comparisons).
ConclusionDesensitization treatment with TPE before live donor renal transplantation in the case of patients with HLA sensitization provides better survival benefits along with monitoring for donor-specific antibodies (DSAs) and other infections, rather than waiting for a compatible organ donor. The data lays out evidence that desensitization treatments can assist overcome HLA incompatibility barriers in live donor renal transplantation.
Renal transplant is the only curative therapy for patients with end-stage renal disease (ESRD). However, long-term outcomes have been dubious and nearly half of the cases lose allografts after a maximum of 10 years post-transplant.1 The significant reason for this is antibody-mediated rejection (AMR) caused by HLA allo-sensitization which occurs due to pregnancy, blood transfusion and exposure to non-self-tissue.2 The pathogenic role of alloantibodies/donor specific antibodies (DSAs) has been established in the 1960s by Patel and Terasaki, using the cytotoxic crossmatch.3 Since then, many advanced strategies for the detection of DSAs have been created, which have worked on the understanding and better administration of the AMR. The introduction of solid-phase assays, for example, the Luminex bead-based assays, permits a more delicate, precise and explicit identification of the DSA.4 HLA-sensitized patients express multiple alloantibodies and crossmatch positivity, resulting in longer waiting times.5 Patients with preformed DSAs are at a higher risk of developing rejections in all solid organ transplant cases.6 The HLA-incompatible (HLAi) living kidney donor transplantation is a popular alternative to expand the donor pool in these patients. The goal of desensitization therapy in such patients is to reduce mean fluorescence intensity (MFI) levels of the DSA, thereby making them potential candidates for transplant. This study was done to evaluate the role of therapeutic plasma exchange (TPE) in HLAi renal transplants.
MethodsStudy populationIn this single-center study, consecutive, ABO-compatible patients with HLA-DSA antibodies, who underwent transplant after desensitization and an equivalent number of ABO- and HLA-matched controls (negative for HLA-DSA/ HLA-compatible patients), who underwent transplant, were included from January 2017 to March 2019 after the ethical committee approval. The demographic details of both the patient and the control group were registered and compared. Written and informed consent was obtained from all subjects. The histocompatibility workup included HLA typing of both recipient and donor, complement-dependent cytotoxicity (CDC) crossmatch, Luminex lysate-based crossmatch (LumXm) and HLA antibody screening. A single antigen bead (SAB) assay was performed in certain cases, wherever possible, along with the donor HLA typing to ascertain the virtual crossmatch.
All CDC-positive patients, irrespective of Luminex-based assays, were excluded from the study. No attempt for desensitization was made for such patients.
All patients positive on Luminex-based assays (LumXm and SAB), but negative with CDC crossmatch, were referred for desensitization.
The HLA incompatibility (HLAi) was defined based on detecting DSA, either through CDC, LumXm, or virtual crossmatch. The DSA ‘reduction’ was defined as the percentage reduction in the immunodominant DSA MFI between pre-and post-apheresis sessions. ‘Severe adverse events were outlined as events occurring during an apheresis session that prompted suspending the procedure. The antibody-mediated rejection (AMR) was assessed based on renal function tests, an unexplained rise in serum creatinine, or an acute graft dysfunction due to the presence of DSA. Patient survival was calculated from the date of transplantation to the date of death. Graft survival (non-censored for death) was calculated from the date of transplantation to the date of irreversible graft failure, signified by a return to long-term dialysis or re-transplantation. The death of the patient, despite having a functional graft, was treated as a graft failure.
The primary outcome of the study was the efficacy of performing an HLAi-kidney transplant (HLAi-KT) after a successful desensitization protocol. DSAs were monitored at least once a week during the desensitization period until the kidney transplant. Thereafter, all patients were followed for a total period of 3 years for kidney dysfunction. All patients on follow-up and having kidney dysfunction were evaluated for DSA at that time. During the overall study period of 3 years, the DSA protocol was performed using lysate-based Luminex bead crossmatch for all the patients for every 0, 3, 6 months, 1 year and yearly thereafter. The secondary endpoints were the safety of the apheresis techniques, the number of severe adverse events, hemodynamic tolerance and the comparison of survival data between the patient and the control group.
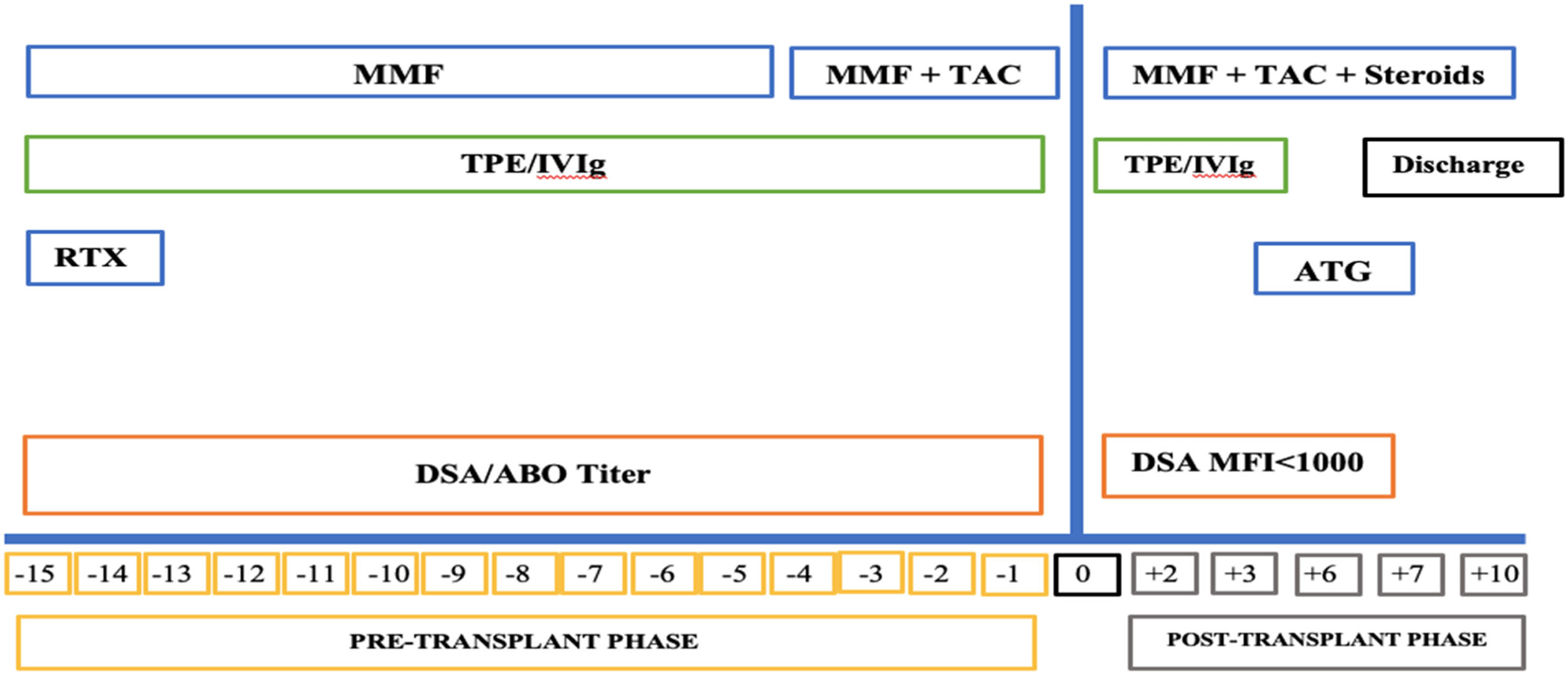
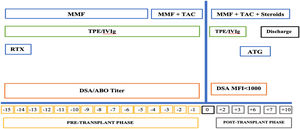
Desensitization and immunosuppressionWritten and informed consent was obtained from the patients who underwent TPE. The highly sensitized patients were treated with a desensitization treatment, as per the hospital protocol, which included TPE and administration of low-dose intravenous immunoglobulin (100 mg/kg per TPE session) (Figure 1). Therapeutic plasma exchange was performed with the use of a centrifuge-driven cell separator, i.e., the Spectra Optia Apheresis System (Lakewood, Colorado 80,215, USA). Escalating numbers of treatments were performed before transplantation based on the level of DSA at the baseline. Momentarily, patients positive for DSA anti-HLA antibodies received between two to four treatment contingents upon initial level of DSA and their response to the earlier two treatments. After completion of the session, the DSA was rechecked to ascertain the DSA ‘reduction’. The goal of the desensitization treatment was the conversion to a negative crossmatch (MFI < 1000) and to sustain the reduction in DSA before transplantation in each patient.
The desensitization protocol included the induction with rituximab (375 mg/m2) in the pre-operative period, followed by tacrolimus (TAC) 0.05 to 0.075 mg/kg every 12 h and mycophenolate mofetil (MMF) 1 g/day in divided doses, from the day of surgery for all the patients. Methylprednisolone was initiated with a 1 gm intravenous dose during the intra-operative phase and continued as 100 mg/day and 80 mg/day on postoperative days (POD) 1 and 2, followed by switching and tapering with prednisolone. A trough level of 8 to 12 ng/ml was maintained for tacrolimus in the first month of the postoperative period and tapered after that to 5 to 8 ng/ml.
The TPE was performed every day. One and a half plasma volumes were exchanged with albumin and saline through a central line. Two units of fresh frozen plasma (FFP) were given at the end of every procedure to prevent coagulation derangement due to dilutional coagulopathy, if any. Anticoagulation was achieved with acid-citrate dextrose (ACD) alone. In all procedures, intravenous calcium replacement was given prophylactically via peripheral line at a dose appropriate to the patient body weight to prevent hypocalcemia due to citrate-related toxicity. All procedure-related adverse events were registered.
Histocompatibility testingAntihemophilic globulin (AHG)-CDC crossmatch (Xmatch)This method involved incubating donor lymphocytes with the patient sera in the presence of the rabbit complement. The blood sample was collected in ACD anticoagulant vacutainers. Lymphocytes were separated using density gradient centrifugation using Histopaque. The AHG-CDCXm was performed using neat and diluted dithiothreitol (DTT) treated patient sera and lymphocytes (B and T cells were separated) of the donor or patient (for autocrossmatch). Appropriate controls (positive and negative) were used. In CDCXm, cell lysis was qualitatively assessed and lysis of more than 20% of the baseline value was considered positive at our institution.
Lysate-based Luminex donor specific crossmatch (LumXm)The LIFECODES Donor Specific Antibody Assay (Immucor Transplant Diagnostics, Inc. USA) kit was used to perform the lysate-based Luminex-based donor-specific crossmatch. Donor lymphocytes isolated from the peripheral blood were used as the source material for the HLA. The isolated cells were solubilized with a non-ionic detergent (lysis buffer, provided in the kit). Following a centrifugation step to remove cell debris and fragments, the lysate was used. The LumXm includes a single blend of Luminex beads. Two of the beads are conjugated with monoclonal antibodies specific for HLA Class I and Class II. This blend of beads, when mixed with lysate, capture the solubilized HLA, making a donor-specific HLA target for antibodies in the serum sample. After capturing donor HLA, the beads were transferred to a filter plate and washed in conjunction with the vacuum manifold. Serum diluted in the specimen diluent was then added and incubated with the beads for 30 min. Following another wash, the diluted anti-human IgG phycoerythrin (PE) conjugate was added to the beads. All incubations were performed on a gently rotating platform in the dark at ambient temperature. After a final 30-min incubation, wash buffer was added to the wells of the plate and acquired on the Luminex platform, and interpretation was performed using MATCH IT Antibody Software. As per the institution protocol, LumXm, MFI less than 1000 was considered negative for Class I and II both, 1000 to 1500 was considered as borderline or weakly positive and over 1500 was considered positive for LumXm.6
HLA antibody screeningThe HLA Class I and Class II screening was performed with LIFECODES LifeScreen Deluxe Kit (Immucor Transplant Diagnostics, Inc. USA). Beads coated with glycoprotein from different donors (pooled beads) were incubated with the recipient serum, followed by the addition of the conjugate (Anti-IgG PE-labeled). The analyte was then acquired as a comma-separated values (CSV) file and imported into LIFECODES MATCH IT Antibody Software for analysis. As per the institution protocol, a test is considered positive for MFI values > 1000 and Negative for MFI values below the cut-off (1000).
Single antigen bead (SAB) assayA freshly obtained, undiluted sera were used to perform the antibody assay. The SAB assay was performed on the Luminex platform using the Lifecodes LSATM Class I and Lifecodes LSATM Class II (Immucor Transplant Diagnostics, Inc. USA) Kits. The beads are designed to qualitatively detect HLA IgG antibodies for both HLA class I and Class II. For this assay, an aliquot of the bead (microspheres coated with HLA Class I and Class II molecules) is incubated with a small volume of test serum sample. The sensitized beads are then washed to remove unbound antibodies, followed by incubation with anti-Human IgG antibodies conjugated to phycoerythrin. For the SAB assay, the signal intensity for each bead is compared to the signal intensity of the lowest ranked locus-specific bead included in the bead preparation. The analysis of the results was performed using the MatchIT Antibody Software. As per the institutional protocol, the bead was considered positive for MFI values over 1000 and negative for values under the cut-off (1000). Furthermore, the positive beads were determined for each locus-specific allele. The donor-specific antibody (DSA) was determined by the corresponding donor HLA typing by the Sanitation Standard Operating Procedures (SSOPs).
Deoxyribonucleic acid (DNA) extraction and HLA typing: The DNA was extracted from peripheral leukocytes by the DNA isolation kit (Qiagen, QIAamp DNA Blood Mini Kit) according to the manufacturer's instructions and it was diluted with 150 ml elution buffer (supplied in kit) and stored at 40C until further analysis. The HLA typing (HLA-A, B, C, DR, DQ, DP, low to medium resolution) was performed using a polymerase chain reaction with the sequence-specific oligonucleotide probe (PCR-SSOP) method on the Luminex 200 platform (Lifecodes HLA SSO Typing Kits, Immucor Transplant Diagnostics, Inc. USA), involving PCR amplification, hybridization, streptavidin phycoerythrin (SAPE) probing and analysis on the Luminex platform to identify the respective allele. The analysis of the results was accomplished using the MatchIt DNA software.
Diagnosis and treatment of patient graft survivalThe antibody-mediated rejection (AMR) was assessed based on renal function tests, an unexplained rise in serum creatinine or an acute graft dysfunction due to the presence of a DSA detected using Luminex-based assays. The graft survival (non-censored for death) was calculated from the date of transplantation to the date of irreversible graft failure signified by a return to long-term dialysis or re-transplantation or date of death. The death of the patient, despite having a functional graft, was treated as graft failure.
Statistical analysisWe used the Kaplan–Meier method to compare the rates of graft survival in the treatment group vs. matched controls. The positive cases were compared within the groups of HLA incompatible recipients based on the level of donor-specific anti-HLA antibody detected using the CDC cross-match, LumXm, HLA antibody screening and single antigen bead assay (in certain cases).
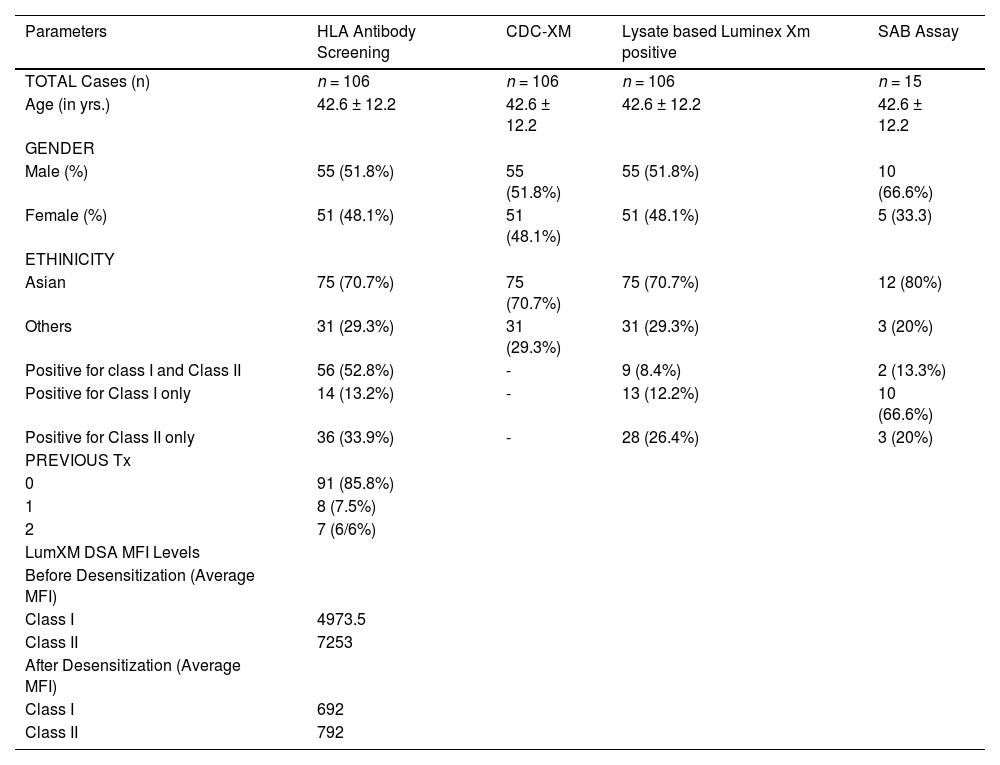
ResultsBetween the period of January 2017 to March 2019, a total of 1473 CDC and Luminex crossmatches were performed at our Institute. Of these, 106 HLAi patients who underwent a kidney transplant after desensitization and an equal number of matched controls were included in the study. The baseline characteristics of HLAi kidney transplant recipients have been detailed in Table 1. Of the 106 HLAi patients, with an average age group of 42.6 years, 52% were male and 48% were females; 71% were Asians and 29% belonged to other ethnicities and racial groups. Ninety-one patients (85.8%) underwent a first-time renal transplant, 8 (7.5%) patients underwent a second renal transplant and 7 (6.6%) patients underwent a third-time renal transplant (Table 1).
Baseline characteristics of HLA incompatible kidney -transplant recipients..
The lysate-based LumXm was positive in 106 patients with an average MFI of 4973.5 in class I and 7253 in class II before desensitization. The HLA antibody screen class I was positive in 14 patients (13.2%), HLA class II, in 36 (33.9%) patients, and both class I and class II were positive in 56 (52.8%) patients (Table 1). The single antigen bead (SAB) assay and the virtual crossmatch were performed in 15 patients whose lysate-based LumXm and HLA antibody screening were positive. The SAB class I was positive in 2/15 patients, the SAB class II was positive in 10/15 patients and the SAB class I and class II (both combined) were positive in 3/15 patients. Characteristics of HLAi kidney transplant patients with HLA-compatible matched control patients (NO DSA group) were also compared and tabulated in Table 2.
Characteristics of HLAi kidney transplant patients with HLA-compatible matched control patients (NO DSA group).
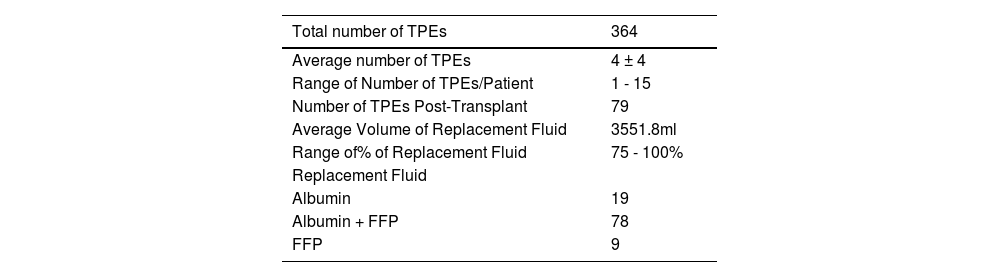
The total number of TPEs performed in these HLA incompatible patients was 364 with an average of 4 ± 4 procedures per patient. A total of 79 patients received one TPE session post-transplantation, as per the clinician's request, prophylactically. The goal of the desensitization treatment was the conversion to a negative crossmatch (MFI < 1000) and to sustain the reduction in the DSA before transplantation in each patient. Post-desensitization, lysate-based LumXm was repeated and a reduction in DSA MFIs was observed with an average MFI of 628 in Class I and 792 in Class II.
The average serum creatinine levels among post-transplant patients were 1.0 mg per deciliter (within normal range) (mg/dl) in 99 (93.39%) patients and 4.95 mg/dl (above normal range) in 7 (6.61%) patients, indicating better functioning of graft and good clinical outcome, post-transplant, in the majority of patients.
The details of the TPE in these HLAi patients are summarised in Table 3. No adverse events were observed during the TPE.
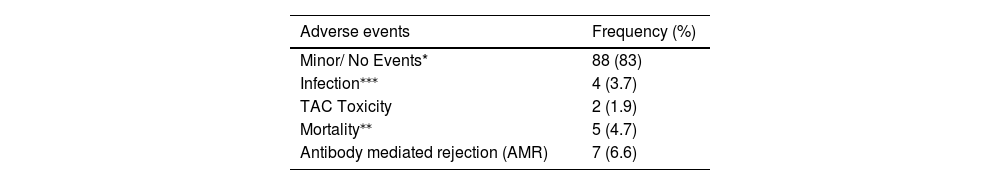
No de novo DSA was observed in the follow-up. However, other complications and post-transplant adverse events are depicted in Table 4.
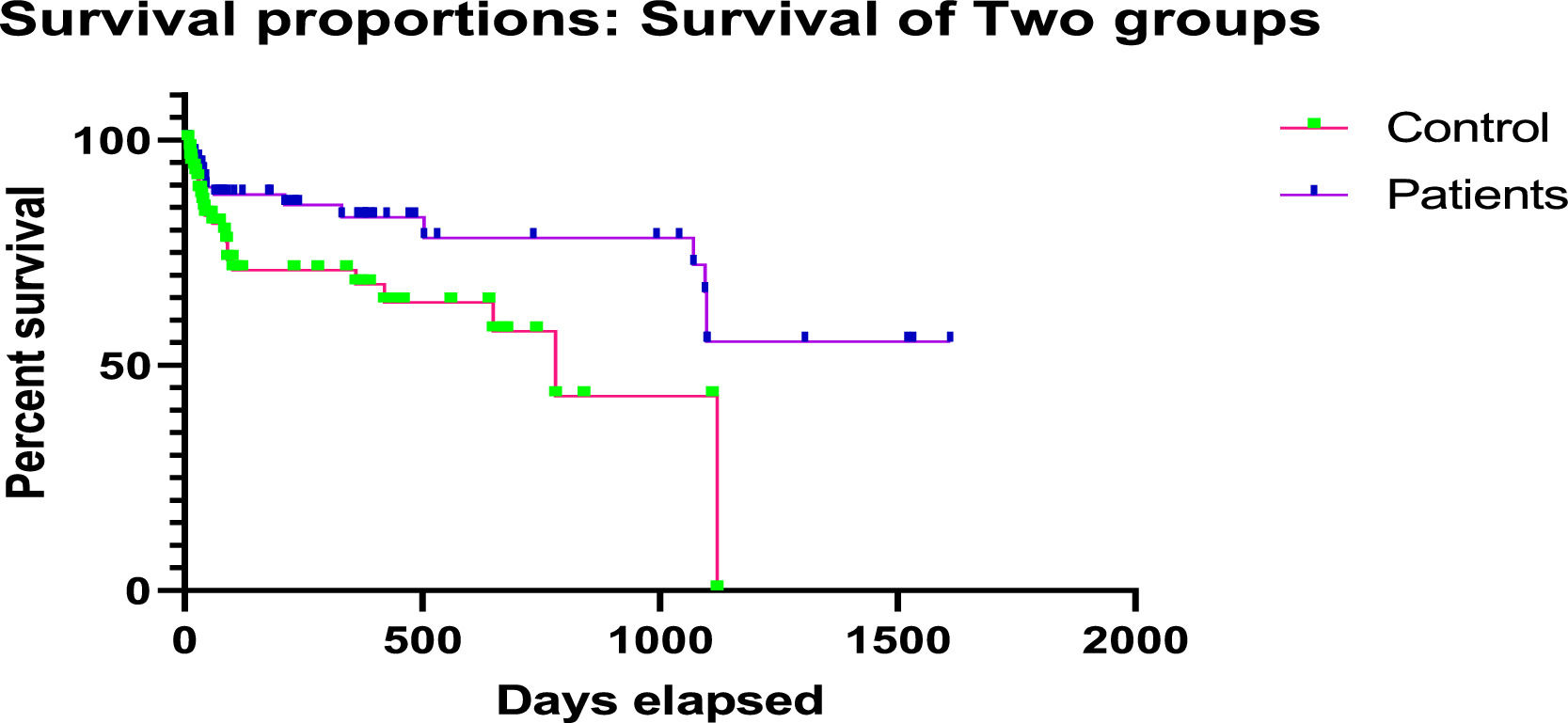
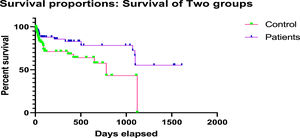
The comparison between the HLA-incompatible patients undergoing desensitization and transplant/treatment group and control group (HLA-compatible) was made using Kaplan–Meier survival curve analysis and survival benefit was observed post-desensitization treatment (Figure 2). The average rate of graft survival at 3 years post-transplant was 95.2% in the treatment group, whereas it was 89.6% in the control group (p < 0.05) (Figure 2).
DiscussionIncreasing evidence suggests that the DSA against the HLA antigen has a predictive and detrimental effect on the renal allograft and overall clinical outcome. A strong correlation between preformed DSA antibodies and the risk of worst graft survival, as well as patient survival, has already been established.7,8 The HLA sensitization is a major problem in approximately 30% of patients waiting for a renal transplant.9 Therefore, a better understanding of preformed DSAs is imperative to plan the desensitization therapy and to improve the selection criteria for kidney allocation in highly sensitized HLA-incompatible patients.
Apheresis techniques, such as the therapeutic plasma exchange (TPE), have been extensively used synergistically with drugs to curb and/or avoid solid organ transplantation rejection. Before transplantation, the TPE is helpful to remove or reduce the titer of pre-formed antibodies to prevent acute rejections. After transplantation, the TPE helps to avoid the AMR. Apheresis can also be used to overcome the ABO incompatibility barrier by depleting the transplant recipient isohemagglutinin (A or B) antibodies.
In our study, we did not find any significant difference in demographic and clinical features in HLA-incompatible patients, when compared with controls, for the presence of DSAs. Our results are in alignment with studies reported by Vo et al.10 and Montgomery et al.,9 in which 16 /20 and 211/215 HLA-incompatible patients underwent successful renal transplant post- desensitization treatment, respectively.
In the present study, we evaluated and compared the survival rates of renal transplant patients with a 3-year follow-up. We report on 106 HLAi patients who underwent desensitization treatment, followed by successful renal transplant after the depletion of DSAs. In a metanalysis, including 1119 patients, the presence of pre-transplant DSAs associated with poorer allograft outcomes has already been established (Mohan et al.).11 The positive CDCXM is a clear contraindication for transplant and has been established previously. Despite a negative flow crossmatch, the CDCXM was found to increase the risk to two times of antibody-mediated rejection and allograft failure, with a relative risk of 1.76, confidence interval (CI:1.13–2.74).11,12
In our study, all patients underwent desensitization and were further investigated to check the levels of DSAs and underwent transplants after the reduced DSA levels (MFI < 1000). The TPE and the desensitization protocol were continued until these optimum levels were obtained before transplant. This was done in consensus with the clinician patient management.
The total number of TPEs performed in HLA-incompatible patients in our study was 364, with an average of 4 ± 4 procedures per patient. A total of 79 patients received one TPE session post-transplantation, as per the clinician's request. Padmanabhan et al. noticed in their study a development of an early AMR in patients who received > 4 TPEs, followed by low-dose IVIg, before transplantation, despite negative crossmatching at the time of transplant. Furthermore, they suggested that such patients may benefit from closer monitoring and more sessions of TPE/IVIg after transplantation.13 Lefaucheur and colleagues reported better outcomes with a regimen of TPE, IVIg and rituximab for the control of the AMR, compared to high-dose IVIg (2 g/kg) IVIg alone.14
The complications arising due to post-renal transplantation infections are considered a major cause of morbidity and mortality, especially in the Asian ethnicity.15 Several reports have found that the BK virus (BKV) allograft neuropathy (1 - 10%) and the cytomegalovirus (CMV) infection contribute significantly to retransplant.16–18 In our study, during the follow-up, monitoring of the patients for infectious markers was performed and 4 cases were found to have an infection, CMV (1 patient), BKV (1 patient) and pneumonia (in 2 patients).
In our study, a total of 5 (4.7%) cases did not survive post-transplant for the period of monitoring. The reasons for death were anaphylaxis, cardiogenic shock and multiorgan failure. There are only a handful of survival studies considering the effect of desensitization in HLA-incompatible patients with a prospective follow-up and risk of graft survival of more than 2 years.9,19 Montgomery et al.9 have reported that the presence of DSA was an important predictor of reduced graft survival and required TPE before transplantation. The authors reported at 3 years a survival rate of 85.7% in HLA-incompatible patients who underwent desensitization.
During the overall study period, desensitization was associated with a significant increase in the rate of patient graft survival, as compared to the rates in the control group. Since desensitization has the potential to significantly reduce the anti-HLA DSA antibodies, it increases access to transplantation, reducing the waiting period for patients.20 Also, promising treatment options for highly sensitized patients, including TPE and immunoadsorption, help in the removal or depletion of the undesirable DSA, making the patient a potential candidate ready for transplant. The levels of DSAs were significantly reduced by the post-desensitization treatment (MFI < 1000) and all the patients had a significant survival benefit. We observed a higher survival rate of 95.2% in the HLA-incompatible treatment group, as compared to 89.6% in the matched control group. Fernández et al. in their study of HLA-incompatible kidney transplantation after desensitization reported a survival rate of 71.9% from a total of 32 patients. The investigators in the study used MFI levels to predict the inefficiency of desensitization and five-year allograft survival of 86% was acceptable, with a low incidence of acute rejection of 17.4%. However, a higher trend toward post-operative bleeding was observed in this study.21 In contrast to these findings, we achieved a higher rate of successful transplantation with better survival and fewer adverse events without any bleeding complications among all the patients post-TPE and desensitization treatment. Tacrolimus toxicity was noted only in 2 cases; total rejections or graft dysfunction (acute and chronic) were reported in a total of 7 (6.6%) cases and death, in 5 (4.7%) patients. Supporting our findings, a review including 21 studies with 725 patients with donor-specific antibodies who underwent kidney transplantation with different desensitization protocols documented an acute rejection rate of 36% with an acceptable short-term patient and graft survival at 2 years (95% and 86%, respectively).22
LimitationsOur study has certain limitations. Firstly, it is a single-center study. This would mean a referral of only a particular set of patients belonging to a specific geographical area. The follow-up for the study is available only for 3 years and was only possible in the treatment group. Also, the SAB assay could not be performed on all patients due to cost constraints and resource unavailability. Secondly, data for the renal biopsy was not available to assess clinical rejections.
ConclusionOur study shows that highly sensitized HLA-incompatible patients, susceptible to higher rejection rates or having a previous history of graft dysfunction due to HLA incompatibility, can derive a maximum survival benefit from desensitization treatment with prospective monitoring of DSAs and other infections. The DSA screening using solid-phase assays does offer higher sensitivity and specificity for HLA antibody detection.
CRediT authorship contribution statementMohit Chowdhry: Conceptualization, Methodology, Supervision, Writing – review & editing. Ayushi Yadav: Writing – original draft, Visualization, Investigation. Vandana Sharma: Writing – original draft, Visualization, Investigation. Soma Agrawal: Writing – review & editing.