The evolutionary conserved link between coagulation and innate immunity is a biological process characterized by the thrombosis formation stimulus of immune cells and specific thrombosis-related molecules. In physiological settings, the relationship between the immune system and thrombosis facilitates the recognition of pathogens and damaged cells and inhibits pathogen proliferation. However, when deregulated, the interplay between hemostasis and innate immunity becomes a pathological process named immunothrombosis, which is at the basis of several infectious and inflammation-related thrombotic disorders, including coronavirus disease 2019 (COVID-19). In advanced stages, alterations in both coagulation and immune cell function due to extreme inflammation lead to an increase in blood coagulability, with high rates of thrombosis and mortality. Therefore, understanding underlying mechanisms in immunothrombosis has become decisive for the development of more efficient therapies to treat and prevent thrombosis in COVID-19 and in other thrombotic disorders. In this review, we outline the existing knowledge on the molecular and cellular processes involved in immunothrombosis, focusing on the role of neutrophil extracellular traps (NETs), platelets and the coagulation pathway. We also describe how the deregulation of hemostasis is associated with pathological conditions and can significantly aggravate a patient's condition, using COVID-19 as a clinical model.
Throughout evolution, most, if not all, multicellular organisms that contain body fluids have developed defense mechanisms to heal wounds by simultaneously limiting fluid loss and eliminating pathogens that enter the host.1,2 This is supported by evidence, which shows that the coagulation system and innate immunity share a common origin dating back to at least 450 million years and have progressively evolved into more complex systems in a parallel manner.2,3
The most ancient known link between hemostasis and inflammation has been established in the horseshoe crab (Limulus polyphemus), an invertebrate that belongs to the subphylum Chelicerata among arthropods.4 In 1964, Jack Levin and Frederik Barry Bang described a highly sensitive system capable of detecting bacterial lipopolysaccharides (LPS) with the use of what became to be known as the Limulus amebocyte, a blood cell lysate from horseshoe crabs that immediately and very efficiently clotted LPS-containing solutions. Subsequent studies on this species revealed that its clotting mechanism was also activated when a trauma to the Limulus exoskeleton occurred, further strengthening the hypothesis that coagulation plays a pivotal role as a defense strategy against infection in invertebrates.2,5,6
Vertebrates, in turn, have an increasingly complex body plan organization, which is often interpreted as an important selective pressure on the evolution of hemostasis, leading to the development of closed circulatory systems and to the organ-specific differentiation of endothelial cells throughout the vascular bed.2,5,6 It has also been shown that the vertebrate coagulation system is closely related to innate immunity: it is known that the intrinsic pathway can both be activated by exposure to pathogenic bacteria (such as Streptococcus pyogenes, Staphylococcus aureus, Salmonella and Escherichia coli) and induce the release of antimicrobial peptides by its co-factor kininogen.7,8 Additionally, prior experiments have demonstrated that the tissue factor, as well as other hemostasis elements, including activated protein C, factor Xa and thrombin, is capable of regulating the host response to an infection by initiating signaling pathways via protease-activated receptors (PARs) on immune cells.9–11
The evolutionary conserved link between coagulation and innate immunity can be seen as a possible explanation for immunothrombosis, a crucial element of intravascular immunity.12 The term immunothrombosis, originally described by Engelmann and Massberg, refers to the formation of thrombi supported by immune cells and specific thrombosis-related molecules with the intent of facilitating the recognition of pathogens and damaged cells, as well as inhibiting pathogen proliferation.12 While hemostasis is a biological process essential to the prevention of blood loss following endothelial injury, thrombosis is generally regarded as its pathological deviation, characterized by the formation of thrombi inside arteries or veins and, thus, causing their occlusion.12 Likewise, immunothrombosis is thought to be a major feature in the pathophysiology of thrombotic disorders.12,13 The coronavirus disease 2019 (COVID-19), in particular, has a prothrombotic tendency proven to be strongly related to harmful alterations in both coagulation and immune cell function.14 Therefore, understanding the underlying mechanisms of immunothrombosis has become decisive for the development of more efficient therapies to treat and prevent thrombosis.
In this review, we outline our current knowledge on immunothrombosis, focusing on the key molecular and cellular processes involved in the crosstalk between hemostasis and innate immunity in physiological settings. We also describe how immunothrombosis can significantly aggravate a patient's condition, using COVID-19 as a clinical model.
Neutrophil extracellular traps (NETs)Neutrophils are the most predominant leukocytes in peripheral blood and they actively participate in the organism's innate defense against pathogens. In 2004, a new antimicrobial activity was described: neutrophils expel a network of chromatin and histone fibers (especially H3 and H4). These structures, called neutrophil extracellular traps (NETs), represent an important strategy to immobilize and kill invading microorganisms.15,16 NETs are a type of trap released by neutrophils on the process of cellular death, termed NETosis.15,16
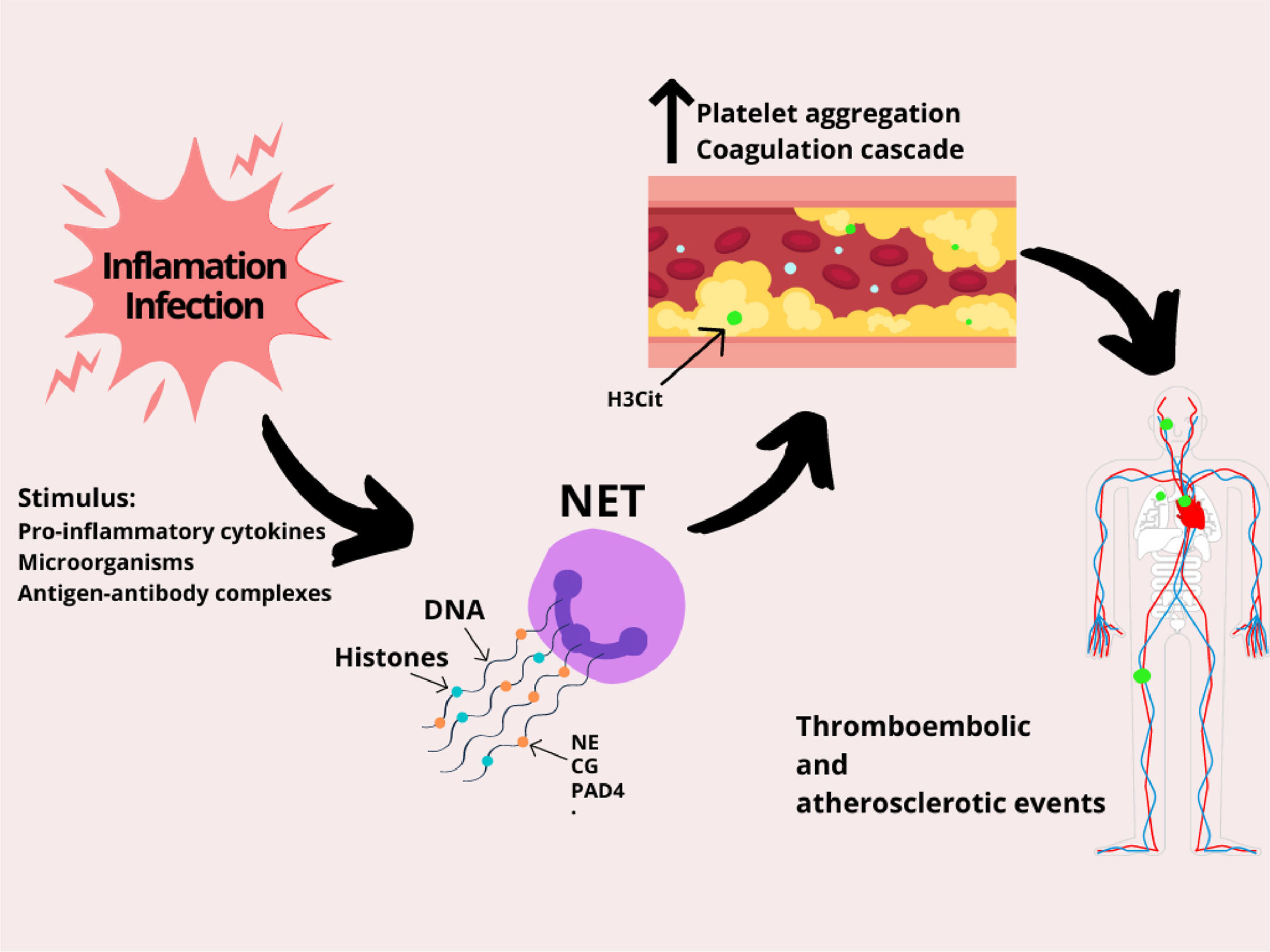
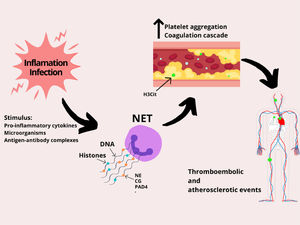
NETs are released as a response to various stimuli, such as microorganisms (infection), pro-inflammatory cytokines (tumor necrosis factor alpha (TNF-α), interleukin 8 (IL-8)), antigen-antibody complexes, platelets and activated endothelial cells (ECs) (Figure 1).16 After receiving these stimuli, membranes dissolve and the nuclear content decondenses into the cytoplasm. This event is followed by the rupture of the plasma membrane and the release of decondensed chromatin with granular proteins in the extracellular space. In this form, the neutrophil can capture the microorganism and release its intracellular content (genetic material, histones and antimicrobial peptides) on the antigen surface, causing the death of the pathogen.16,17 Three of the enzymes that take part in this process will be mentioned throughout the text: neutrophil elastase (NE), peptidyl-arginine deiminase 4 (PAD4) and cathepsin G (CG). Although the initial observation indicated that the NET genesis is an important tool to fight the pathogen, evidence suggests that these structures are also at the center of various pathological states, such as thrombosis formation, atherosclerosis and autoimmune processes.15
Specifically, thromboembolic events begin with a change in blood flow caused by: disturbances of venous blood flow, activation or dysfunction of the vascular endothelium and hypercoagulability. Initially, a local hypoxia and endothelial cell activation is triggered, which releases factor von Willebrand (VWF) and P-selectin, responsible for activating platelet aggregation. The platelets, in turn, recruit the tissue factor (TF), that increases thrombin generation, catalyzing the conversion of fibrinogen to fibrin, which is responsible for the thrombus stabilization. The NET is the additional factor for the whole process of thrombosis, as it exacerbates platelet and endothelial cell activation and promotes fibrin formation.17,18
The histones that form the NET body have a high affinity for phospholipids and, in addition, their connection to membranes results in the formation of pores and an influx of ions, increasing intracellular calcium levels.18 As a result, the endothelial cells are hyperactivated, which favors thrombus formation. In addition, the purified histones bind to the surface of platelets, likely via Toll-like Receptors (TLRs), promoting an intracellular calcium influx, which generates platelet hyperactivation.18 Furthermore, the NET contains active NE enzymes and Cathepsin G, which potentiate platelet aggregation through proteolytic activation of platelet receptors.17,18
The NET stimulates fibrin formation and deposition, as it stimulates extrinsic and intrinsic coagulation pathways.17,18 The NE enzyme cleaves the factor inhibitor of the TF pathway, thereby increasing factor Xa activity.17,18 In addition, the NE binds to factor XII and stimulates fibrin formation through the intrinsic coagulation pathway.17,18 Lastly, through thrombomodulin, histones inhibit the activation of protein C, which is a plasmatic anticoagulant and therefore, the stimulus to the fibrin generation will be greater.17,18
From a clinical point of view, many studies are being performed on a new drug that would directly inhibit the formation of NETs, thereby decreasing thromboembolic events. The drug is called DNAse1, which was initially observed in bacteria that evolved not to suffer the action of NETs.18 Its action consists of inhibiting the PAD4 enzyme, responsible for the decondensation of chromatin and, consequently, the formation of the NET.19–21 Recent studies have used the biomarker citrullinated histone H3 (H3Cit) through immunostaining to quantify the presence of NETs. The observed results were the expressive amount of the H3Cit in thromboembolic events and also the best prognosis when using DNAse1 in addition to the standard treatment.19,20 In conclusion, it is of utmost importance that more studies clarify if the NET formation in thromboembolic events can be avoided and, from a clinical point of view, if DNAse1 can be seen as a new therapeutic drug for thrombolysis in the daily medical practice.
Role of NETs in platelet and coagulation activation. Inflammation and infection stimulate neutrophils to release NETs. However, the NET composition (DNA strands, histones, and enzymes) dysregulates blood hemostasis by stimulating the activation of platelets and the coagulation pathways, which may lead to several thromboembolic complications, such as ischemic stroke, pulmonary embolism, heart attack and deep vein thrombosis. Abbreviations: neutrophil elastase (NE), cathepsin G (CG) and peptidyl-arginine deiminase 4 (PAD4).
Platelets, also known as thrombocytes, are tiny anucleated cells, derived from megakaryocytes, produced in the bone marrow. After leaving the bone marrow, platelets circulate in the blood for approximately seven to ten days, following which they are eliminated by macrophages in the spleen and liver.22 The most important function of the platelets is to maintain hemostasis, initiating the coagulation cascade. However, evidence points to a key role of platelets in inflammatory and immune responses, in the same manner as the neutrophils.22–24
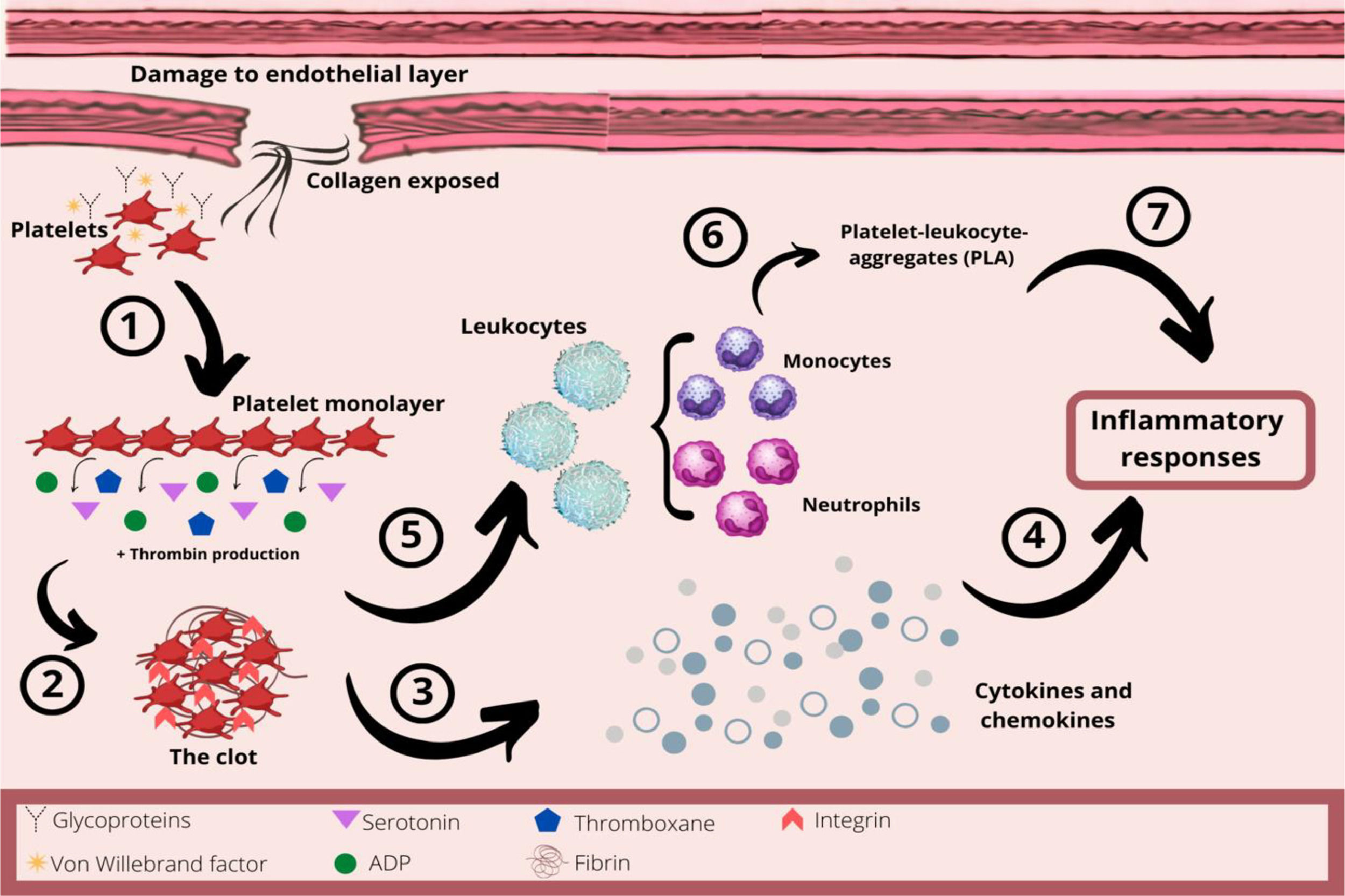
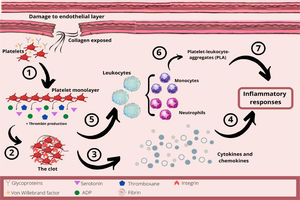
In hemostasis, damage to the endothelial layer exposes collagen from the subendothelial space, which interacts with platelets through their glycoproteins and von Willebrand factor (Figure 2). The platelets change their shape and adhere to the site of the injury. This collagen exposure leads to platelet adhesion and formation of the platelet monolayer. These activated platelets recruit other circulating platelets by secreting aggregatory mediators, such as serotonin, adenosine diphosphate (ADP) and thromboxane, and by producing thrombin. Therefore, due to activated GPIIb/IIIa integrins and the thrombin released, the platelets aggregate and form a three-dimensional structure, the clot.22,23,25,26
The role of platelets in hemostasis and inflammatory response. 1- After damage to the endothelial layer, platelets interact with collagen from the subendothelial layer. This collagen exposure causes platelet adhesion and formation of the platelet monolayer. 2- These activated platelets recruit other circulating platelets by secreting mediators, such as serotonin, ADP and thromboxane, as well as producing thrombin. Therefore, the platelets aggregate and form a three-dimensional structure, the clot. 3- Platelets are an important source of chemokines and cytokines at the site of inflammation. 4 - These chemokines and cytokines will be important in the acute inflammatory process. 5 – The platelet-mediated leukocyte recruitment is initiated by a close interaction with leukocytes, inducing the activation of integrins and increasing the adhesion of leukocytes to the endothelial layer. 6 - Neutrophils and monocytes can also interact with endothelium-adherent platelets or directly with platelets, forming platelet-leukocyte-aggregates (PLAs) which are recruited to the damaged endothelial layer. 7 - Thus, platelets orchestrate the inflammatory response by regulating the adhesion of innate immune cells to the inflammatory process.
In addition, it is important to note that platelets have secretory granules, which are essential to normal platelet function. Among the three types of platelet secretory granules, the α–granules, dense granules and lysosomes, the α–granule is the most abundant and it allows the platelet to increase its surface area by 2- to 4-fold, upon platelet stimulation and/or spreading. The α–granule function derives from its contents. The content of the α–granule includes both membrane-bound proteins, expressed on the platelet surface, and soluble proteins, that are released into the extracellular space. There is evidence that secreted α–granule proteins function in coagulation, inflammation, atherosclerosis, antimicrobial host defense, angiogenesis, wound repair and malignancy.27,28
As already explained, following an injury with its subsequent vasoconstriction, platelets are the first immunomodulatory cells to arrive and seal damaged blood vessels by aggregation, forming a thrombus.23,24 Furthermore, platelets promote inflammatory activity by their close interaction with leukocytes, as platelet-mediated leukocyte recruitment is initiated by the linking of the platelet P-selectin to the leukocyte P-selectin glycoprotein, inducing the activation of integrins (β1 and β2), and increasing adhesion of the leukocyte to the endothelial layer.23–26
Furthermore, activated platelets are characterized by P-selectin exposure, which is critical for both hemostasis and platelet–immune cell interactions. The platelet P-selectin is a ligand for the non-activated neutrophil PSGL1 and the P-selectin–PSGL1 bond enables the neutrophil attraction to sites of thrombus formation under low shear stress. However, the capability of platelets to induce neutrophil activation is not limited to the P-selectin–PSGL1 interaction. Platelet ɑ-granules contain a set of chemokines, which are potent stimulators of neutrophil activity. Among the most abundant are the platelet factor 4 CXCL4 (PF4) and the neutrophil-activating peptide-2 CXCL7 (NAP2).29,30
Platelets also secrete the high mobility group box 1 (HMGB1), which can activate both the receptor for advanced glycation endproducts (RAGE) and toll-like receptor 4 (TLR4) on neutrophils and, thus, initiate the NET formation. Finally, upon platelet δ-granule secretion, the ADP and ATP are released, which can activate neutrophil purinergic receptors. Other molecules which can mediate the neutrophil activation upon platelet granule secretion include the transforming growth factor beta (TGFβ), cluster of differentiation 40 ligand (CD40L) and interleukin 1 beta (IL-1β).30–32
Thus, the interactions between platelets and neutrophils are crucial for inflammation and immunity. Currently, the platelet–neutrophil crosstalk is well characterized, both in physiologic and pathologic conditions, in which the thromboinflammatory response is often described as “a vicious cycle”.31
Furthermore, inflammation can cause endothelial dysfunction due to high levels of pro-inflammatory cytokines (IL-1, IL-6 and TNF), as well as ferritin. In addition to direct platelet activation, activated neutrophils can trigger the activation of the plasma coagulation cascade via the TF expression on their surface, as well as the secretion of TF-positive microvesicles. This results in the production of thrombin and fibrin generation, which significantly enhances platelet–neutrophil bond formation and mutual activation.14,31
Thus, in response to pathogens and tissue damage, there is a coordinated intravascular coagulation, recently called 'immunothrombosis'. In other words, this term has been used to describe the interaction between macrophages, polymorphonuclear cells, platelets, coagulation factors and immune effector proteins, forming thrombus in the microvasculature to identify pathogens and mechanically restrict their spread. Therefore, another distinct feature of platelet–neutrophil interactions in immunothrombotic conditions is the increased potency of neutrophils to form NETs, thus facilitating coagulation and forming a positive feedback loop.31,33
This allows platelets and immune cells to form a physical barrier that prevents the spread of pathogens and activates the immune system. Since platelets carry the transcripts for all pathogenic toll-like receptors, during certain bacterial infections, platelets are able to induce prothrombotic events, secrete cytokines, chemokines and antimicrobial peptides, leading to the sequestration and destruction of bacteria. In addition, platelets are also known to envelop certain viruses such as HIV, hepatitis C virus and encephalomyocarditis and to interact with bacteria, such as Staphylococcus Aureus and Staphylococcus Pneumoniae.22–24
CoagulationBlood clotting is a mechanism which is necessary to prevent blood from leaking out of the vessels, in addition to promoting the regeneration of the injured tissue.12,33–35 This process is triggered by the exposure of the TF which, in normal situations, is not encountered in contact with the bloodstream,12,35 but rather in the extravascular cells, so that the coagulation cascade only starts when tissue damage occurs.12 The TF is expressed by fibroblasts, pericytes, epithelial cells, monocytes and neutrophils, in addition to circulating in the blood in the form of microparticles.33,34 When in contact with the bloodstream, the TF forms a complex with the activated factor VII (FVIIa), activating the extrinsic pathway of the coagulation cascade.
The release of the TF is connected with the increase of cytokines, chemokines and the adhesion factor in the blood, which is an innate defense mode of the body.33
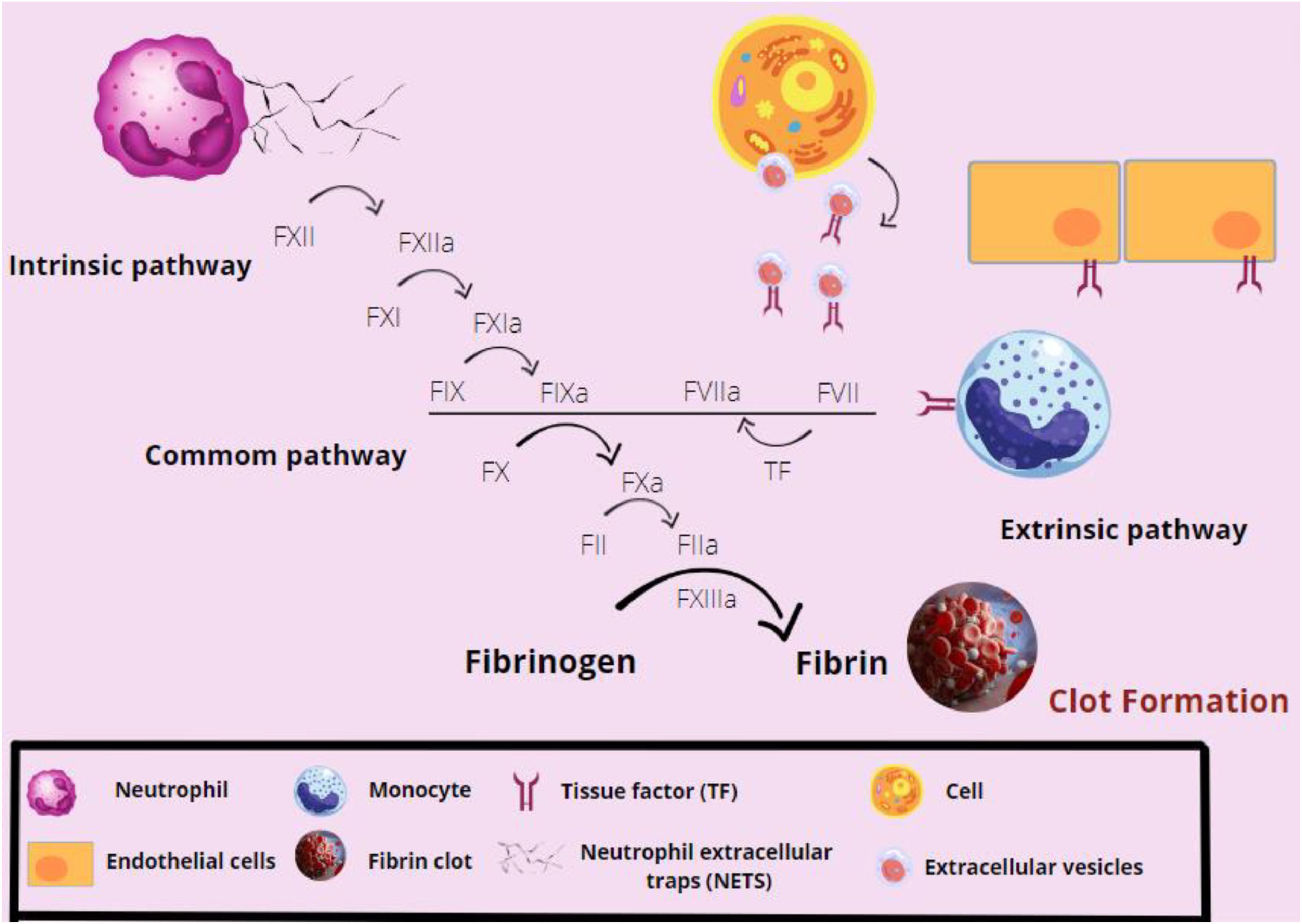
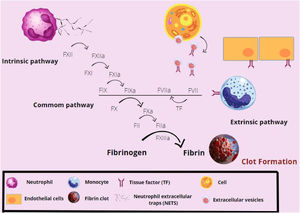
The complex, then formed by the TF-FVIIa, leads to the activation of the factor X (factor Xa), which will promote thrombin formation from prothrombin, enhancing factor X activation (feeding back to the cascade). Most of the pro-inflammatory functions of factor Xa and thrombin are mediated by protease receptors (PARs), that intermediate coagulation and inflammation. PARs are expressed by cells, such as platelets, leukocytes and endothelial cells. The factor X activates PAR1 and PAR2, while thrombin activates PAR3, with both activations leading to the release of inflammatory cytokines expressed by the cells. In addition to its expression on endothelial cells, the tissue factor can be expressed on activated monocytes, extracellular vesicles, NETs and polyphosphates released by activated platelets.33,34 These cytokines attract monocytes and neutrophils, in addition to acting on the leukocyte adhesion and on the expression of receptors by antigen-presenting cells. They also become capable of altering vascular permeability, favoring inflammatory processes.33,34
The thrombin is also responsible for the conversion of fibrinogen into fibrin, resulting in the formation of a stable clot.12,34 Fibrin also plays a role in bacterial restraint, as bacteria may be trapped inside the formed clot. Furthermore, fibrin promotes chemotaxis and the adhesion of leukocytes as macrophages, dendritic cells and neutrophils.33 The factor XIIIa prevents fibrin degradation and improves the conditions for adhesion of leukocytes to vessels, in addition to acting on monocytes, improving their structure and, consequently, phagocytic functions.33,34 The coagulation process is summarized in Figure 3.
Overview of the coagulation cascade activation. The factor XII (FXII) is activated by the neutrophil extracellular traps (NETs), initiating the extrinsic pathway of coagulation. In sequence, the FXIIa activates the FXI, which acts in the activation of the hyperactive factor IX (FIX). The FIXa will act together with the intrinsic pathway FVIIa, which has been sensitized by the tissue factor (TF) present on the surface of cells, such as monocytes and endothelial cells, to activate the FX. The FXa will form fibrin from fibrinogen and the fibrin network will be stabilized by factors XIa and XIIIa. Thus, the clot is formed.
An endogenous route of blood anticoagulation, that includes the tissue factor pathway inhibitor (TFPI), activated protein C and antithrombin,12 in addition to a fibrinolytic system, prevents the dissemination of a systemic coagulation process. The fibrinolysis is mainly modulated by the tissue plasminogen activator (tPA) and the plasminogen activator inhibitor (PAI-1), which will degrade fibrin clots33 and restore the intravascular flow.34
Besides acting as natural anticoagulants or fibrinolytic agents, these proteins also play a role in the immune response. Studies have shown that the TFPI has destructive properties against different bacteria and fungi, such as the E. coli, S. aureus, and Candida albicans.32 The protein C activates the PAR1 and, in turn, increases the release of pro-inflammatory cytokines by monocytes, which cause neutrophil chemotaxis.33,35 Finally, plasmin, in addition to its fibrinolytic effect, facilitates the activation and migration of leukocytes.35 The tissue plasminogen activator (t-PA) is capable of activating neutrophils and of promoting the release of pro-inflammatory cytokines by monocytes.35 The urokinase plasminogen activator (u-PAR) receptor plays an important role in the leukocyte adhesion and migration.33
Thromboinflammation in COVID-19When the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters endothelial cells, it promptly causes direct vascular injury and damages airway epithelial cells, the most commonly injured tissue due to its many ACE2 receptors.36–38 Additionally, the entry of the SARS-CoV-2 results in cell death, ultimately reducing the ACE2 activity. Thus, there is a predominance of angiotensin II activity, which, in turn, promotes a hypertensive and inflammatory effect, in addition to stimulating the activation of the coagulation cascade via tissue factor (TF).39
Moving onto the mechanisms by which SARS-CoV-2 promotes cell damage and organic dysfunction, it is important to have in mind that inflammatory and immunologic responses closely relate to hemostasis, since the virus lessens innate response and triggers hyperinflammation, followed by hypercoagulability and immunothrombosis.40 Innate immune cells, such as monocytes and neutrophils, recognize molecular patterns associated with pathogens (PAMPs) via their pattern recognition receptors (PRRs). These PRRs recognize the SARS-Cov-2 RNA, culminating in the production of type I interferons (IFN-α and IFN-β), which block viral replication; however, SARS-CoV-2 probably inhibits the interferon response, since it is not usually detected in the plasma of severe COVID patients.41 This inhibition delays the antiviral response and promotes viral replication. Then, a dysregulated and delayed type I IFN response will trigger, along with cytokines and chemokines released from pneumocytes due to cytopathic effects, an infiltration of monocytes and neutrophils in the lung parenchyma. In turn, innate cells produce pro-inflammatory cytokines (IL-1β, IL-6 and tumor necrosis factor alpha (TNFα)), which amplify the recruitment of more innate immune cells and activation of the complement system, resulting in a hyperinflammatory state and cytokine storm that characterizes severe cases of COVID-19.40 Subsequently, the hyperinflammation contributes to the intravascular coagulopathy, as it promotes endothelial dysfunction, once platelets are also activated by the release of some proinflammatory cytokines, such as the IL-6.42 All these components mentioned above interact to form clots in a process known as immunothrombosis, which can be considered a helpful mechanism of intravascular immunity, acting as a defense barrier inside vessels whereby the interaction between innate immune cells (monocytes and neutrophils) and coagulation mechanically inhibits the spread of the pathogen.14,40 However, when immunothrombosis is uncontrolled, it causes unbalanced activation of the coagulation cascade, leading to the microthrombus formation, which predominantly affects the microvasculature and causes microangiopathy.14,38,42 Taken together, these conditions augment the inflammatory response, which later results in extensive organ failure.37,43
As for innate immune cells and immunothrombosis, both monocytes and neutrophils have an indubitable role in the procoagulant profile of COVID-19 patients. Ten percent of monocytes respond to the SARS-Cov-2 infection, which depends on antiviral antibodies.41 The monocyte NLRP3 inflammasome, which detects the K+ efflux, activates the caspase-1 leading to the inflammatory death, known as pyroptosis, and release of pro-inflammatory cytokines, such as the IL-1β and IL-18, and lactate dehydrogenase (LDH).14,38,41 Both the IL-1 and LDH correlate with the development of severe diseases.41 Regarding neutrophils, these are also hyperactivated during SARS-Cov-2 infections.14,43 However, it is known that in inflammatory conditions, overwhelmed neutrophils are prone to release NETs during NETosis, which are webs constituted of chromatin and microbicidal proteins that help trap the virus spread, even though they have the inflammatory potential to occlude vessels and initiate immunothrombosis, as they also trap monocytes, more neutrophils and platelets.43 The NETs, in turn, lead to systemic alterations, stimulating the intrinsic pathway, once it activates the FXII, activating endothelial cells, platelets and complement system and inactivating endogenous anticoagulants due to proteases in their constitution.36,40 The complement system contributes to the recruitment of neutrophils as well, as some of its proteins, such as the C3a and C5a, can upregulate the activity of the TF, activating even more neutrophils, which release the IL-8.38,42
Lastly, platelets, which are also hyperactivated in COVID-19 patients, also play an important role in this disease pathogenesis.14,40,43 The platelet activation triggers inflammation, which makes it release alpha granule stored molecules that contribute to the recruitment of more monocytes and neutrophils. Monocytes, then, interact with platelets and originate platelet-monocyte complexes, which induce the TF expression in monocytes. In turn, hyperactivated neutrophils form platelet-neutrophil aggregates, inducing the formation of more NETs and fueling the cycle of a pro-thrombotic state.36 Moreover, the NETs, coagulation and endothelial activation result in an increased von Willebrand factor (VWF) and in an increased P-selectin. The VWF is a glycoprotein released by endothelial cells and platelets that recruits leukocytes at sites of vascular inflammation. Therefore, the VWF increases the platelet adhesion to the endothelium and fibrin formation. The P-selectin is a cell adhesion molecule that enables the recruitment of platelets and leukocytes and can also induce the monocyte TF expression, leading to a procoagulant phenotype.14,36,40,42
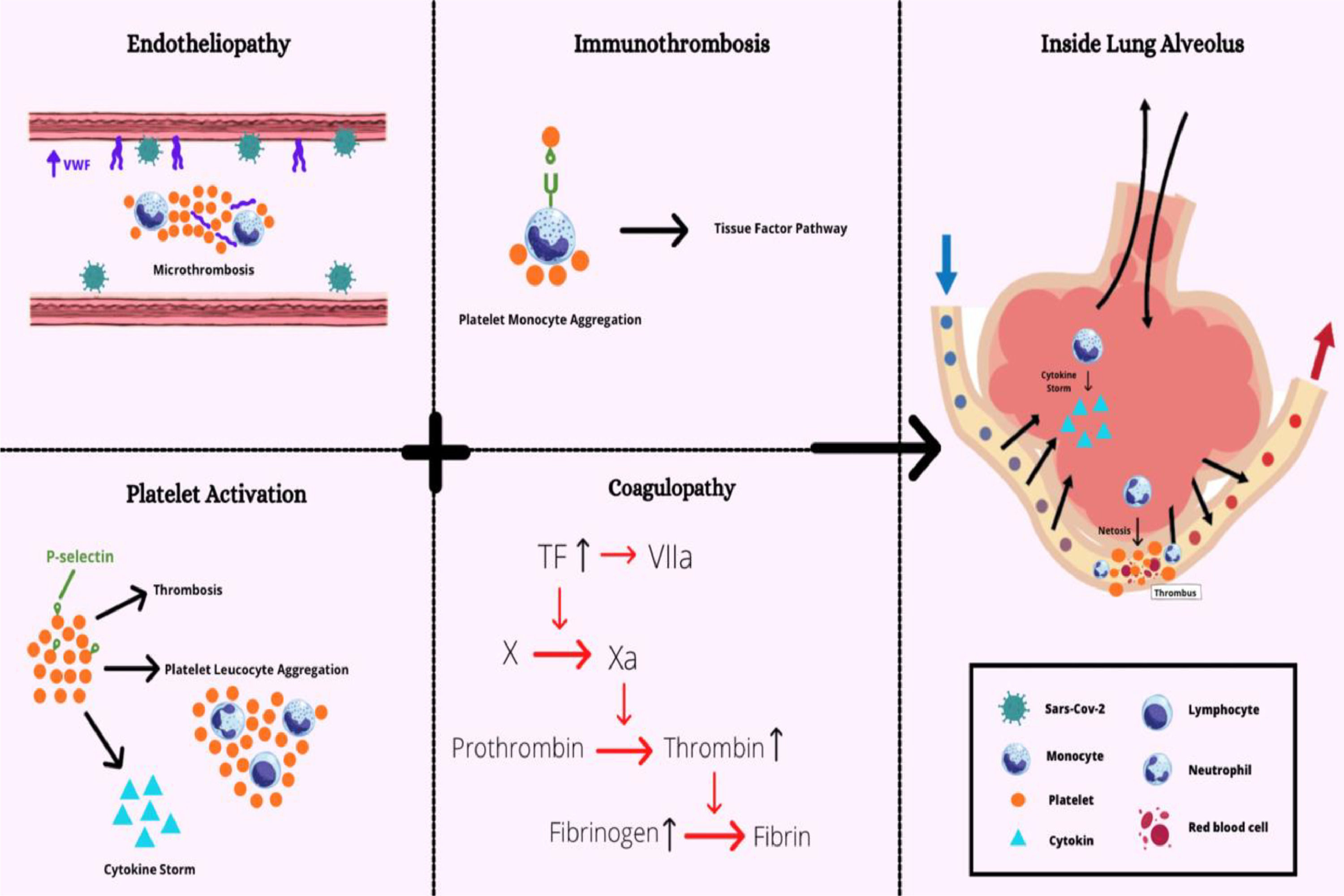
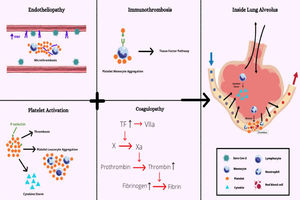
Altogether, it is possible to infer that many factors propagate the procoagulant state during the SARS-CoV-2 infection, which leads to severe complications. The endotheliopathy, platelet hyperactivation, monocyte and neutrophil-induced cytokine storm and coagulopathy, as summarized in Figure 4, seem to be intrinsically interlaced in the COVID-19 physiopathology, which demonstrates the importance of reaching a deep understanding of these mechanisms.
Overview of main factors involved in immunothrombosis due to COVID-19. Increased von Willebrand factor (VWF) released by endothelial cells and platelets recruit leukocytes during inflammation. The P-selectin enables the recruitment of platelets and more leukocytes and induces monocyte TF expression. The TF, in turn, stimulates the activation of the coagulation's cascade. The coagulopathy and platelet activation result in the endotheliopathy, leading to immunothrombosis when all factors are combined.
While hemostasis is a biological process essential to the prevention of blood loss following an endothelial injury, thrombosis is generally regarded as a pathological deviation, characterized by the formation of thrombi inside arteries or veins and, thus, causing their occlusion. Likewise, thromboinflammation is thought to be a major feature in the pathophysiology of thrombotic disorders, which represent a leading cause of death worldwide. COVID-19, in particular, has a prothrombotic tendency proven to be strongly related to harmful alterations in both coagulation and immune cell function. Therefore, understanding the crosstalk between hemostasis and inflammation has become decisive for the development of more efficient therapies to treat and prevent thrombosis.
Author contributionsA.C. De Nardi, A. Coy-Canguçu, A. Saito, M.F. Florio, and G. Marti equally contributed to the study by performing the data collection and drafting the manuscript. G.R. Degasperi oversaw the data collection and revised the manuscript. F.A. Orsi was responsible for the study conceptualization, oversaw the data collection and revised the manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.