Elevated serum progranulin (PGRN) levels have been associated with a wide range of different human malignancies. However, data available on the role of PGRN in hematological malignancies are limited.
MethodsMeasurement of the PGRN level in serum of adult de novo acute myeloid leukemia (AML) patients using enzyme-linked immunosorbent assay (ELISA) was performed.
ResultsThe mean serum PGRN level in AML patients was higher than that in controls (346.08 pg/ml ± 64.46 vs 155 pg/ml ± 63 respectively; p = 0.001). After a mean duration of follow-up equaling 140 days, patients with high serum PGRN (i.e., higher than 370.5 pg/ml) had inferior overall survival (OS) in comparison to patients with low serum PGRN (i.e., lower than 370.5 pg/ml) (OS = 25% vs 60.7%, mean survival = 107 days vs 256.5 days, p = 0.007). On the other hand, remitted patients on day 28 with high serum PGRN (i.e., higher than 307.5 pg/ml) did not differ from those with low serum PGRN (i.e., lower than 307.5 pg/ml) regarding disease-free survival (DFS) (DFS = 78.6% vs. 87.5%, mean survival = 301.3 days vs. 283.5 days, p = 0.789). Moreover, the serum PGRN level was associated with inferior OS (p = 0.024) on multivariate analysis.
ConclusionAdult de novo AML patients have elevated serum PGRN levels and a high PGRN level is associated with an inferior OS.
The growth factor progranulin (PGRN) has significant biological effects in different types of cancer. This protein is a regulator of tumorigenesis because it stimulates cell proliferation, migration, invasion, angiogenesis, malignant transformation, resistance to anticancer drugs and immune evasion.1 In the extracellular matrix, PGRN binds to receptors, resulting in either activation of a signal transduction pathway or engulfment into the cell. Several studies have shown PGRN involvement in the binding of Sortilin, which promotes tumor cell proliferation, migration and survival and induces drug resistance.2 The PGRN activity is associated with p44/42 mitogen-activated protein kinase, as well as phosphatidyl-inositol 3-kinases signaling pathways. In addition, PGRN may stimulate the formation of the tumor stroma. Tumor necrosis factor and ephrin type-A receptor 2 were suggested as potential PGRN facilitators.3
In breast cancer, PGRN has been implicated in tumorigenesis and resistance to anti-estrogen therapies for estrogen receptor positive breast cancer. Previous pathological studies showed that PGRN is expressed in invasive ductal carcinoma, but not in normal mammary epithelial tissue, benign lesions or lobular carcinoma.4 In Rheumatoid Arthritis patients, the levels of circulating serum PGRN have been measured and were found to be higher than those in age-matched healthy controls.1 The PGRN levels were higher in the serum of patients with lymphoid malignancies than in healthy controls. High PGRN plasma levels were found to be strongly associated with adverse risk factors in chronic lymphocytic leukemia (CLL) patients, including unmutated IGHV (immunoglobulin heavy chain variable region) status, expression of CD38 and ZAP-70, and poor risk cytogenetics (11q-, 17p-), suggesting that PGRN is a novel, robust and independent prognostic marker in CLL.5 Furthermore, high serum PGRN levels were associated with poor prognosis in patients with diffuse large B cell lymphoma (DLBCL).6 Moreover, our group proved recently that high serum PGRN level may be used as a predictor of increased relapse risk in adult de novo acute lymphoblastic leukemia (ALL) patients.7 The aim of the study was to measure levels of PGRN in the serum of adult patients with acute myeloid leukemia (AML) and to correlate its serum levels with prognosis and clinical outcome.
MethodsThis study was conducted on 80 subjects (40 adult de novo AML patients and 40 age- and sex-matched healthy persons) who were attending the Clinical Hematology and Oncology Unit, Internal Medicine Department, Ain Shams University, during the period from June 2018 to June 2019. Patients under the age of 16 years, with relapsed AML and a history of other malignant disease, rheumatological disease or neurodegenerative disease were excluded from the study. Diagnosis of AML was established by history taking, clinical examination, complete blood count, metabolic profile, bone marrow (BM) morphological examination, immunophenotyping and genetic studies (karyotyping and fluorescent in situ hybridization). Radiographic investigations for assessment of extramedullary disease were performed. Cerebrospinal fluid analysis (cytology) was performed in the case of the identification of M4 and M5 French – American – British (FAB) subtypes or if manifestations suggesting central nervous system (CNS) infiltration existed. Patients were followed up for a maximum period of 13 months and survival and remission status were assessed on day 28 and at the end of the study. Baseline patient characteristics are summarized in Table 1.
Baseline patient characteristics and their correlation with serum PGRN level:
| Variable | Mean (range) | Correlation with mean serum PGRN level in all patients (346 ± 64 pg/ml) | ||
|---|---|---|---|---|
| r | P | |||
| Age, years | 43.93 ± 17.45 (17 – 75) | 0.274 | 0.087 | |
| TLC, cell x 109/L | 39.35 ± 67.96 (0.80 – 335) | 0.063 | 0.698 | |
| Hemoglobin, gm/dL (range) | 7.62 ± 1.94 (4 – 13) | -0.012 | 0.942 | |
| Platelets, cell x 109/L | 58.20 ± 51.5 (4 – 220) | -0.033 | 0.838 | |
| LDH, U/L | 924.88 ± 687.39 (147 – 2890) | -0.185 | 0.253 | |
| BM blasts, % (range) | 64.30 ± 23.82 (8 – 99) | 0.140 | 0.389 | |
| N (%) | Mean serum PGRN level (pg/ml) | P | ||
| Gender | Male | 25 (62.5%) | 325.16 ± 60.77 | 0.050a |
| Female | 15 (37.5%) | 365.00 ± 63.15 | ||
| Comorbidityb | Positive | 9 (22.5%) | 354 ± 75 | 0.650 |
| Negative | 31 (77.5%) | 344 ± 61.44 | ||
| EMD | CNS | 0 (0%) | NA | 0.069 |
| HSM + LN | 20 (50%) | 364.55 ± 65.64 | ||
| Negative | 20 (50%) | 327.60 ± 59.19 | ||
| FAB | M1-2 | 24 (60%) | 337.92 ± 73.09 | 0.747 |
| M3 | 5 (12.5%) | 338.00 ± 56.32 | ||
| M4-5 | 9 (22.5%) | 362.33 ± 47.45 | ||
| M6 | 1 (2.5%) | 382.00 | ||
| M7 | 1 (2.5%) | 400.00 | ||
| Cytogenetic riskc | Low | 15 (37.5%) | 328.40 ± 50.24 | 0.177 |
| Intermediate | 21 (52.5%) | 349.38 ± 72.83 | ||
| High | 4 (10%) | 395.00 ± 45.28 | ||
| Chemotherapy regimen | 3+7 | 28 (71.8%) | 346.64 ± 63.58 | 0.665 |
| Pethema | 4 (10.3%) | 316.25 ± 32.80 | ||
| Palliatived | 7 (17.9%) | 349.57 ± 81.44 | ||
Abbreviations: TLC: total leukocytic count; LDH: lactate dehydrogenase; EMD: extramedullary disease; CNS: central nervous system; HSM: hepatosplenomegaly; LN: lymphadenopathy; FAB: French-American-British; N: number; PGRN: Progranulin; NA: not applicable.
Comorbidities were: hypertension (4 patients), diabetes mellitus (1 patient), chronic viral hepatitis (3 patients) and ischemic heart disease (1 patient).
The serum PGRN level was measured in patients at diagnosis and controls using the Human Progranulin (PGRN) enzyme-linked immunosorbent assay (ELISA) kit (The Cloud-Clone Corp.™, USA). This assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for human PGRN has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any PGRN present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked monoclonal antibody specific for the human PGRN is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of PGRN bound in the initial step. The color development is stopped and the optical density (O.D.; Absorbance) is measured at 450 nm. The amount of PGRN in each sample is determined by plotting the O.D. value against the corresponding concentration on the standard curve.
Definitions and statistical analysisThe overall survival (OS) was defined as the length of time from the date of diagnosis to the date of death due to any cause. The disease-free survival (DFS) was defined as the length of time between achieving complete remission and relapse or last follow-up. Data were collected, revised, coded and entered into the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges, when parametric. Furthermore, qualitative variables were presented as numbers and percentages. The comparison between groups regarding qualitative data was done by using the Chi-square test or Fisher exact test when the expected count in any cell was less than 5. The comparison between independent groups regarding quantitative data with parametric distribution was made by using the One-Way ANOVA. The Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Areas under the curves (AUC) and receiver operating characteristic (ROC) curves were used for dichotomizing variables regarding total death events and relapse events following complete remission (CR). The Kaplan–Meier analysis was used to assess the impact of variables on OS and DFS by using the log rank test. Only variables found to be significant in the univariate analysis were included in the multivariate analysis. The multivariate analysis was performed using the Cox regression analysis. The confidence interval was set to 95% and the margin of error accepted was set to 5%. Therefore, the p-value was considered significant if ≤ 0.05.
Compliance with ethical standardsA written informed consent has been obtained from all the study participants. Approval of the study by the Ethics Committee Board, Faculty of Medicine, Ain Shams University was obtained. The study conformed to the stipulations of the Declaration of Helsinki.
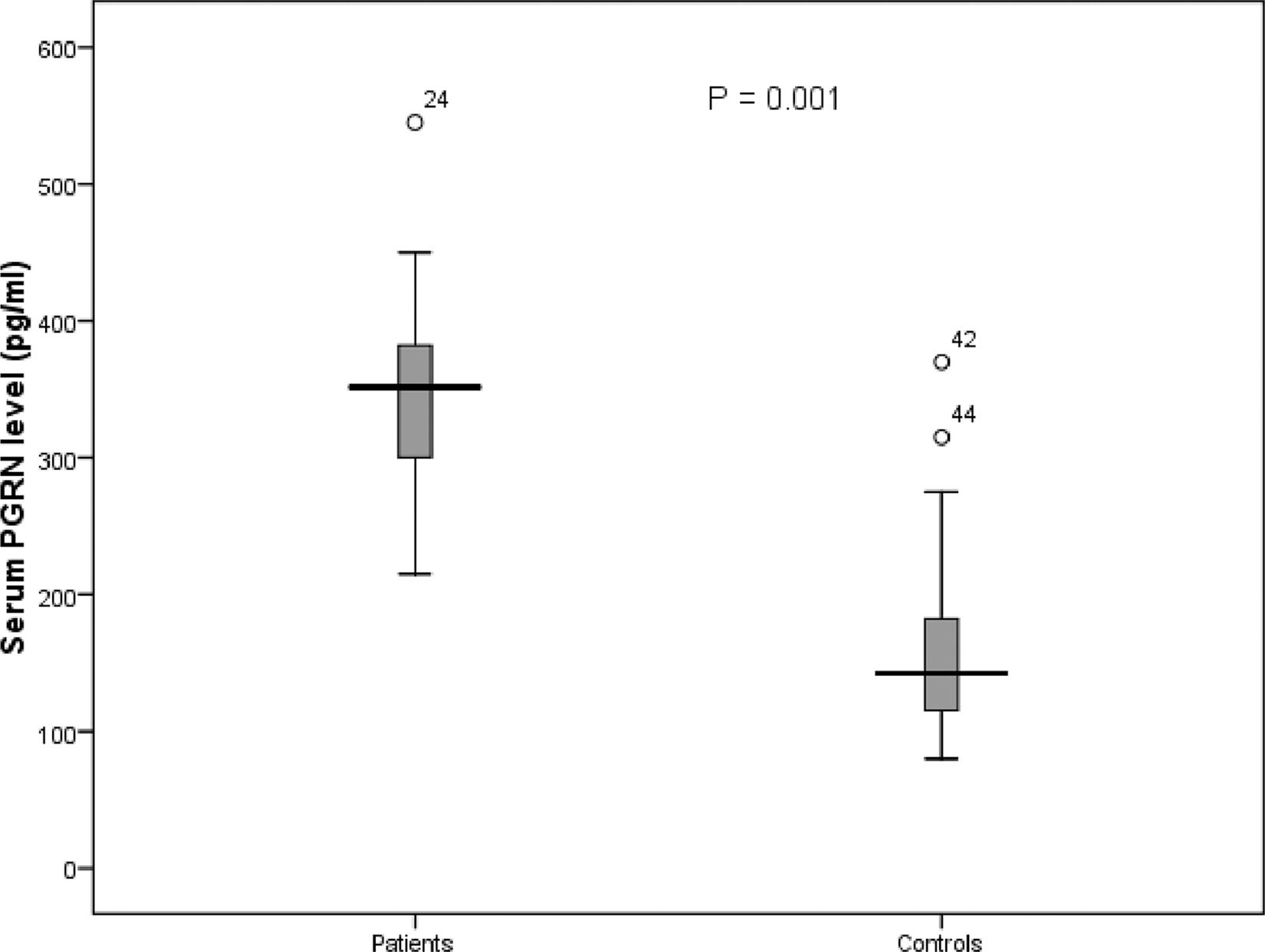
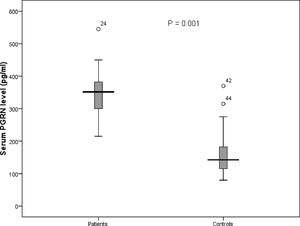
ResultsSerum PGRN level in patients and controls and its correlation with other variablesThe serum PGRN was higher in patients than in controls (mean = 346 ± 64 pg/ml (range = 215 – 545) vs. 155 ± 63 pg/ml (range = 80 – 370), p = 0.001) (Figure 1). The PGRN level did not correlate with age (p = 0.087), total leukocytic count (p = 0.698), hemoglobin (p = 0.942), platelet count (p = 0.838), serum lactate dehydrogenase (p = 0.253) and BM blast percent (p = 0.389) (Table 1). Moreover, the mean serum PGRN level did not differ between patients with comorbidities and those without (p = 0.650), FAB subtypes (p = 0.747), patients with extramedullary disease and those without (p = 0.069) and cytogenetic risk groups (p = 0.177) (Table 1). In contrast, the serum PGRN was higher in female patients than in males (p = 0.050) (Table 1).
Outcome of patientsOn day 28, 29 (72.5%) patients were alive and 11 (27.5%) were dead. Of the 29 living patients, 22 (75%) were remitted and 7 (25%) were resistant. At the end of the study, 4 of the 22 remitted living patients relapsed and 9 patients died, resulting in a mortality rate of 50% (total deaths = 20). Of these, an additional 9 died, 6 were resistant to induction chemotherapy and 3 relapsed after achieving remission. Other causes of death were septicemia (10 patients) and disseminated intravascular coagulopathy due to AML M3 (1 patient).
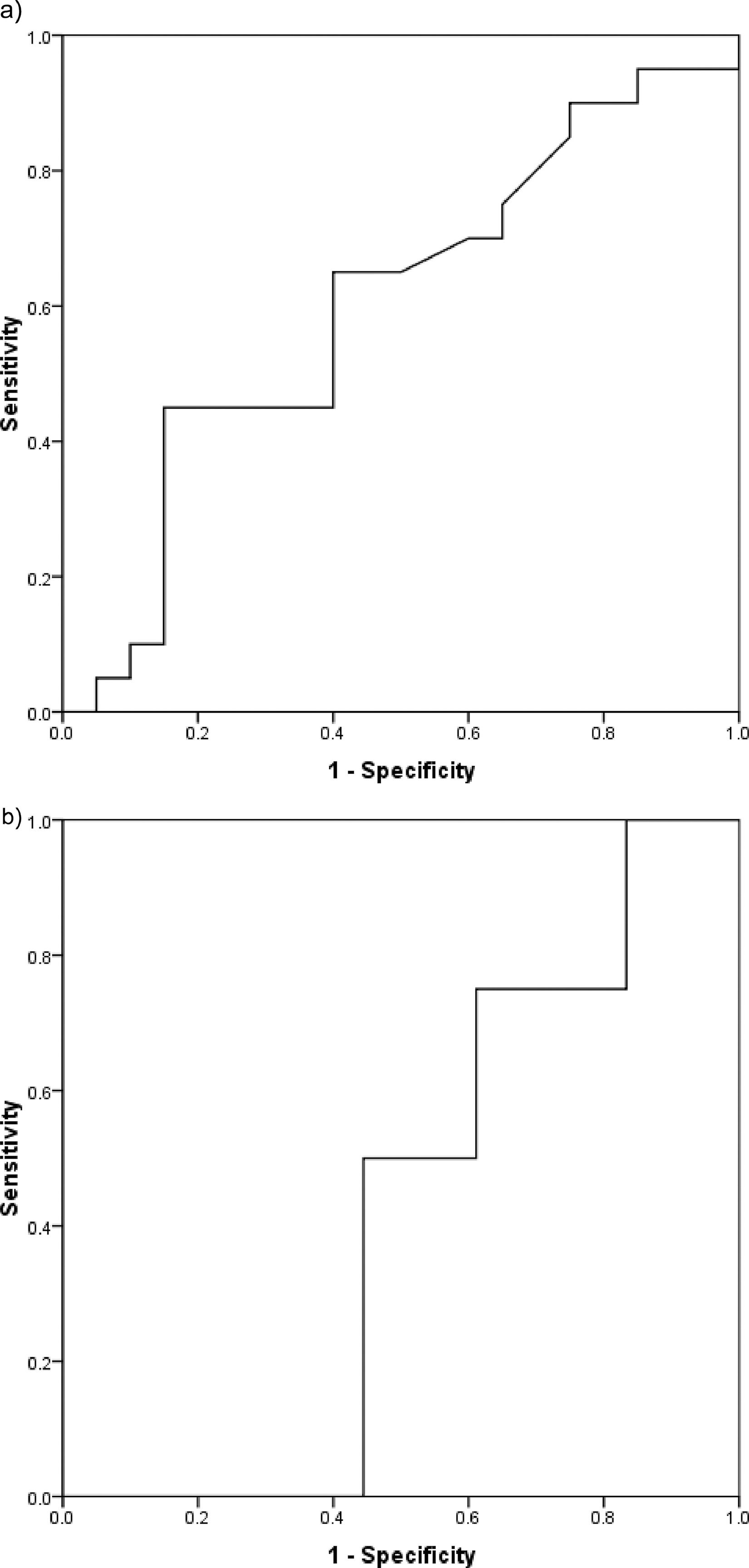
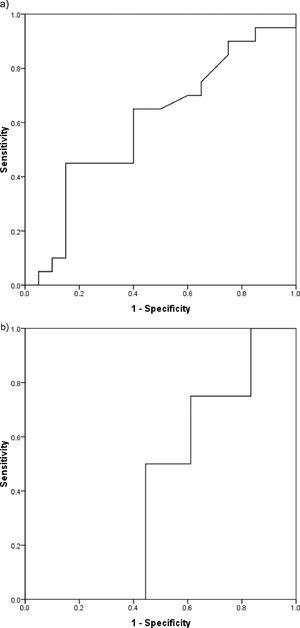
Determination of the optimum cutoff value for dichotomizing serum PGRN levelThe optimum cutoff value for dichotomizing the serum PGRN level regarding mortality in the whole patient cohort was 370.5 pg/ml (AUC = 0.600, 95% confidence interval (95% CI) = 0.420–0.780, sensitivity = 45%, specificity = 85%, p = 0.279) (Figure 2a). The optimum cutoff value for dichotomizing the serum PGRN level regarding relapse in the 22 patients who achieved CR was 307.5 pg/ml (AUC = 0.417, 95% CI = 0.168–0.665, sensitivity = 75%, specificity = 38.9%, p = 0.610) (Figure 2b).
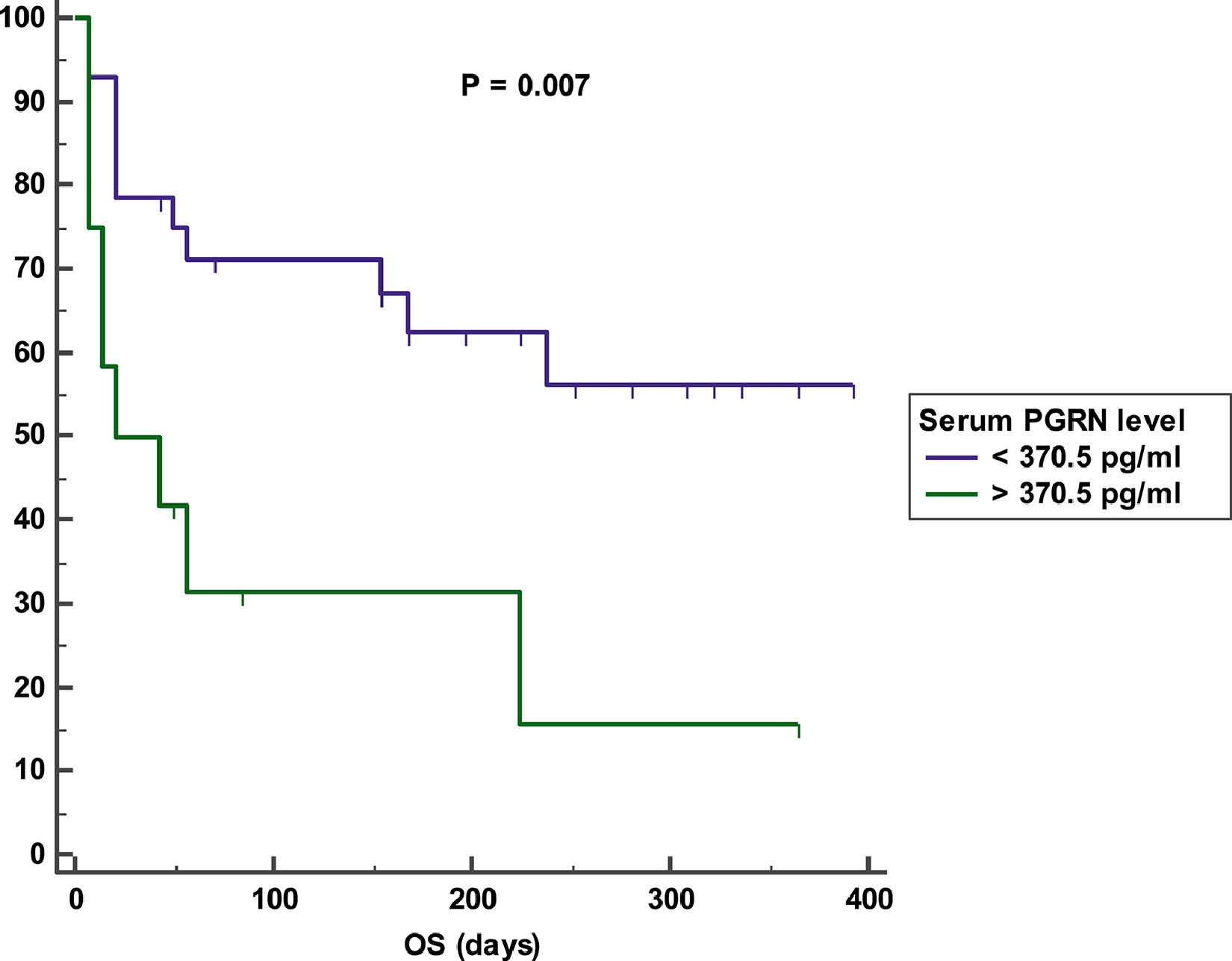
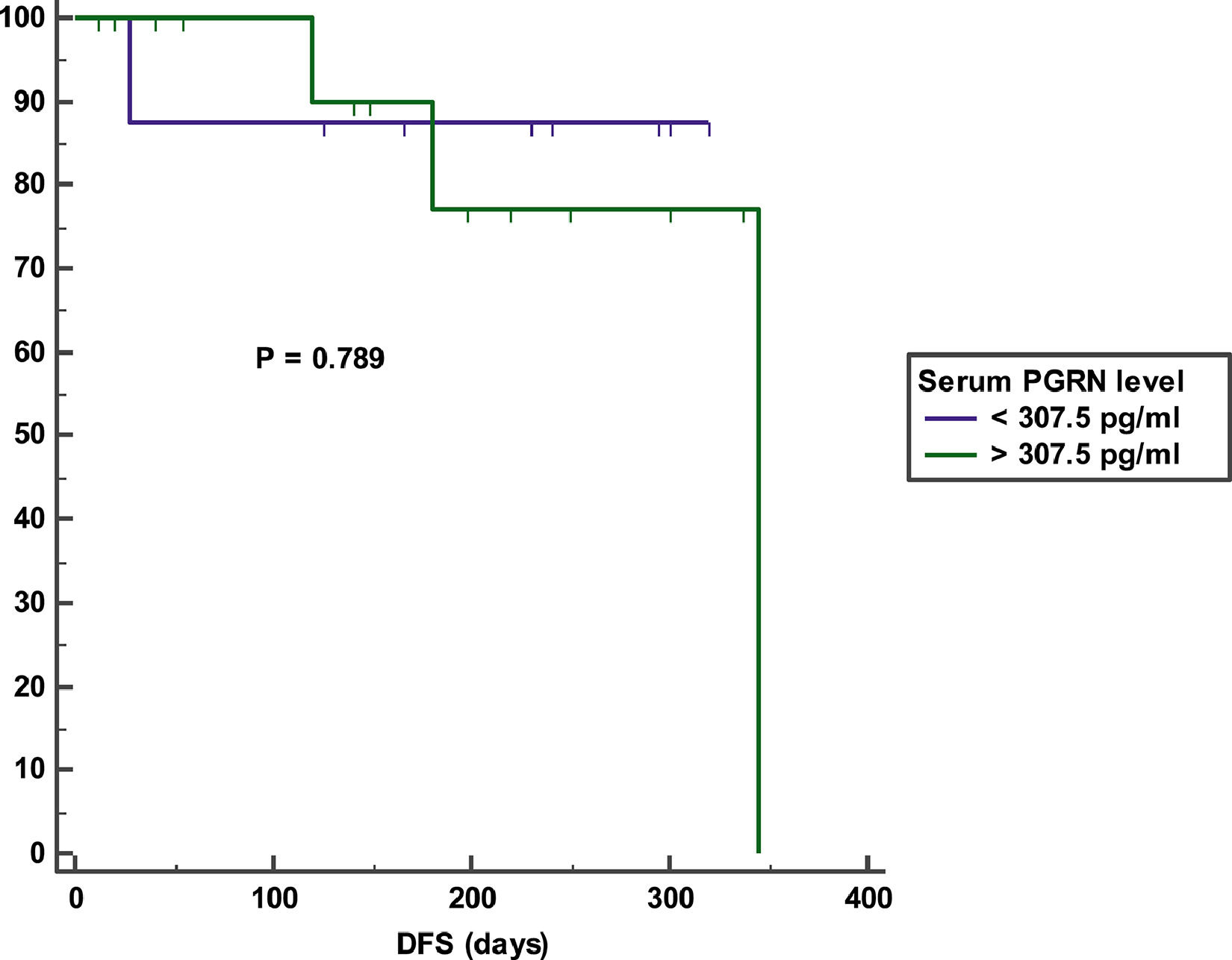
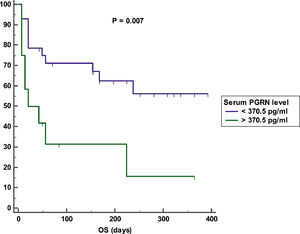
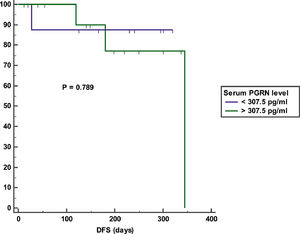
Impact of serum PGRN on outcomeAfter a mean duration of follow-up equaling 140 days (7 – 392 days), patients with a high serum PGRN (i.e., higher than 370.5 pg/ml) had an inferior OS in comparison to patients with a low serum PGRN (i.e., lower than 370.5 pg/ml) (OS = 25% vs 60.7%, mean survival = 107 days vs. 256.5 days, 95% CI = 26.9 - 187.2 vs. 193.9 - 319.2, p = 0.007) (Figure 3). On the other hand, remitted patients on day 28 with a high serum PGRN (i.e., higher than 307.5 pg/ml) did not differ from those with a low serum PGRN (i.e., lower than 307.5 pg/ml) regarding DFS (DFS = 78.6% vs. 87.5%, mean survival = 301.3 days vs 283.5 days, 95% CI = 235.5-367.1 vs 216.6-350.4, p = 0.789) (Figure 4).
On univariate analysis of variables in the OS, age > 38.5 years, presence of comorbidities, total leukocytic count > 145 × 109/L, lactate dehydrogenase > 599.5 U/L, high-risk cytogenetics and serum PGRN level > 370.5 pg/ml were associated with inferior OS (p = 0.006, p = 0.012, p = 0.013, p = 0.005, p = 0.028 and p = 0.007, respectively) (Table 2). On multivariate analysis, the total leukocytic count, lactate dehydrogenase and serum PGRN levels were associated with an inferior OS (p = 0.036, p = 0.009 and p = 0.024, respectively) (Table 2).
Univariate and multivariate analyses of impacts of serum PGRN level and other variables on OS:
| Variable | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N | N of surviving patients (%) | Mean survival (days) | P | HR | 95% CI | P | ||
| Gender | Male | 25 | 13 (52%) | 224.7 | 0.563 | |||
| Female | 15 | 8 (46.7%) | 184.3 | |||||
| Age | >38.5 years | 23 | 8 (34.8%) | 136 | 0.006* | 1.459 | 0.381-5.584 | 0.582 |
| <38.5 years | 17 | 12 (70.6%) | 304.6 | |||||
| Comorbidity | +ve | 9 | 2 (22.2%) | 100.3 | 0.012* | 2.151 | 0.691-6.691 | 0.186 |
| -ve | 31 | 18 (58.1%) | 245.3 | |||||
| EMD | +ve | 20 | 9 (45%) | 187.8 | 0.581 | |||
| -ve | 20 | 11 (55%) | 230.3 | |||||
| TLC | >145 × 109/L | 3 | 0 (0%) | 25.7 | 0.013* | 4.760 | 1.108-20.447 | 0.036* |
| <145 × 109/L | 37 | 20 (54.1%) | 229.8 | |||||
| Hemoglobin | >6.3 gm/dl | 31 | 14 (45.2%) | 187.7 | 0.117 | |||
| <6.3 gm/dl | 9 | 6 (66.7%) | 268.6 | |||||
| Platelets | >35 × 109/L | 23 | 12 (52.2%) | 220.9 | 0.767 | |||
| <35 × 109/L | 17 | 8 (47.1%) | 196.3 | |||||
| LDH | >599.5 U/L | 24 | 7 (29.2%) | 142.6 | 0.005* | 5.928 | 1.571-22.368 | 0.009* |
| <599.5 U/L | 16 | 13 (81.2%) | 321.1 | |||||
| BM blasts | >87.5% | 8 | 4 (50%) | 191 | 0.679 | |||
| <87.5% | 32 | 16 (50%) | 218.8 | |||||
| FAB subtype | M1-M2 | 24 | 12 (50%) | 204.8 | 0.053 | |||
| M3 | 5 | 4 (80%) | 315 | |||||
| M4-M5 | 9 | 4 (44.4%) | 145.4 | |||||
| M6 | 1 | 0 (0%) | 224 | |||||
| M7 | 1 | 0 (0%) | 7 | |||||
| Cytogenetic risk | Low | 15 | 11 (73.3%) | 308.4 | 0.028* | 1.259 | 0.459-3.448 | 0.655 |
| Intermediate | 21 | 8 (38.1%) | 156.9 | |||||
| High | 4 | 1 (25%) | 36.8 | |||||
| PGRN level | >370.5 pg/ml | 12 | 3 (25%) | 107 | 0.007* | 3.913 | 1.193-12.831 | 0.024a |
| <370.5 pg/ml | 28 | 17 (60.7%) | 256.5 | |||||
Abbreviations: EMD: extramedullary disease; TLC: total leukocytic count; LDH: lactate dehydrogenase; BM: bone marrow; FAB: French-American-British; PGRN: progranulin; N: number; HR: hazard ratio; 95% CI: 95% confidence interval.
AML is characterized by clonal expansion of undifferentiated myeloid precursors, resulting in impaired hematopoiesis and BM failure. Although many patients with AML have a response to induction chemotherapy, refractory disease is common and relapse represents the major cause of treatment failure.8 The growth factor PGRN has significant biological effects on different types of cancer. Elevated PGRN levels have been associated with a wide range of different human malignancies, such as carcinomas of the breast, ovary, liver, kidney, prostate and brain. Furthermore, high PGRN expression levels, as detected in the tumor itself or in the peripheral blood, have been linked to an aggressive phenotype and poor prognosis in breast cancer, glioblastoma and ovarian cancer.9 However, data on the role of PGRN in hematological malignancies are limited. In patients with CLL, high PGRN plasma levels were strongly associated with adverse risk factors, including unmutated IGHV status, expression of CD38 and ZAP-70, and poor risk cytogenetics. The PGRN was prognostic for the OS, suggesting that it is a robust and independent prognostic marker in CLL that can be easily measured by the ELISA.5 In multiple myeloma, it has been demonstrated that the PGRN promotes cell survival and confers resistance to dexamethasone treatment in vitro.10 Furthermore, in patients with malignant lymphoma, the serum PGRN was higher than that in normal controls.6 Additionally, our group demonstrated recently an association between a high serum PGRN level and an increased relapse risk in adult de novo ALL patients.7
In line with data from studies in normal individuals and patients with breast and ovarian cancer, we found that the PGRN can be easily and reliably measured in the peripheral blood, employing a commercially available ELISA assay.9,11 Göbel et al. compared PGRN messenger ribonucleic acid (mRNA) concentrations in immune-magnetically purified CLL cells with PGRN protein plasma levels in the same patients and observed a significant correlation.5 Furthermore, cell culture studies using purified CLL cells revealed a time-dependent secretion of PGRN into the culture supernatant, providing circumstantial evidence that PGRN concentrations measured in the plasma indeed reflect the amount of PGRN production in the leukemic cells derived from individual patients. In our study, PGRN levels in healthy participants were in the range from 80 to 370 pg/ml, with a mean value of 155 pg/ml, while in the patients the range was from 215 to 545, with a mean value of 346 pg/ml, indicating a significant difference between the control and patient groups. This is to some extent similar to what has been reported by Yamamoto et al., who examined the concentration of the PGRN in the plasma from 100 normal individuals and 254 malignant lymphoma patients by ELISA and found a higher PGRN in patients than in the control group.6 Additionally, Göbel et al. examined the concentration of the PGRN in plasma from 31 normal individuals and 131 CLL patients by ELISA and found that CLL patients exhibited elevated PGRN levels, as compared to controls, with no apparent differences for age and sex.5
In our study, the correlations between the PGRN level and patient age, hemoglobin concentration, total leukocytic count, platelet count, lactate dehydrogenase and BM blast percentage at the time of diagnosis were insignificant. This is not in line with Göbel et al. who found a clear positive association in patients with CLL between increasing leukemic cells and PGRN plasma levels.5 This discrepancy may be explained by the chronic nature of CLL, in contrast to the acute rising of blast count in AML and to the limited number of patients in our study. However; in our study, PGRN levels were higher in patients with a high tumor burden (patients suffering from hepatosplenomegaly and/or lymphadenopathy), when compared to the patients with a low tumor burden (patients without extramedullary disease), with a tendency towards significance. In our study, PGRN levels were higher in female patients, when compared to male patients. Nicholson et al. also observed a higher plasma PGRN level in females than in males.12 However, Göbel et al. did not find a difference between male and female patients with CLL regarding serum PGRN levels.5 This can be explained by the different disease nature in our study than in the Göbel et al. study.5 In our study, PGRN levels in patients with low-risk cytogenetics were the lowest and in patients with high-risk cytogenetics were the highest, but this was insignificant. This is not in line with Göbel et al., who observed strong association between high PGRN plasma levels and high-risk cytogenetics in patients with CLL.5 This can be explained by the low percentage of patients with high-risk cytogenetics in our study (10 %), in comparison to the percentage of patients with high-risk cytogenetics in the Göbel et al. study (33%).5
In our study, we evaluated the prognostic value of the PGRN in our AML patients. After a mean duration of follow-up of 140 days for our patients, Kaplan-Meier analyses revealed an inferior OS in the high versus low PGRN patient subgroups. Furthermore, the DFS was lower in the high versus low PGRN patient subgroups, but this was insignificant. This is in line with Göbel et al., who reported differences in terms of the OS between the two groups in patients with CLL and Yamamoto et al., who also observed a strong association between the PGRN level and OS in patients with DLBCL.5,6 Moreover, the PGRN level was an independent risk factor for an inferior OS in the multivariate analysis in our study. However, this study is not in agreement with our other study performed on ALL patients, whose serum PGRN level correlated with an inferior DFS, but not with the OS.7 This discrepancy can be attributed to the different pathophysiology of AML and ALL. Notably, although the cytogenetic risk category is a well-documented prognostic factor in AML patients,13 it did not influence the OS in the multivariate analysis of variables in our study. Such a finding can be attributed to the low numbers of patients in our cohort and to the short duration of the follow-up. The multivariate analysis for the DFS was not performed in our study because the serum PGRN was not found to impact the DFS in the univariate analysis.
We can conclude from our study that the PGRN level is high in adult de novo AML patients and that it may correlate with the tumor burden in AML. In addition, AML patients with a high PGRN level have an inferior OS and therefore, this can be used as a prognostic marker for the OS in AML. We recommend studying the exact pathophysiological role of the PGRN in AML. Furthermore, we encourage analyzing the prognostic impact of the PGRN level in relapsed/refractory AML patients. Moreover, we suggest correlating the PGRN level with other prognostic markers, e.g., the FLT-3 mutational status, p53 abnormalities and NPM1.
This research did not receive any specific grants from funding agencies in the public, nor not-for-profit sectors