People living with human immunodeficiency virus (PLWH) still face high morbidity and mortality resulting from lymphoma.
AimTo describe a population of PLWH and lymphoma in a Chilean public hospital and compare the overall survival (OS) with a previously reported cohort from the same institution.
MethodsRetrospective single-center cohort study. All the patients diagnosed between 2010 and 2017 were included. Demographic and clinical variables were obtained from medical records. The overall survival (OS) was estimated in treated patients from diagnosis until death or October 2020. The OS was then compared with a cohort of patients diagnosed between 1992 and 2008.
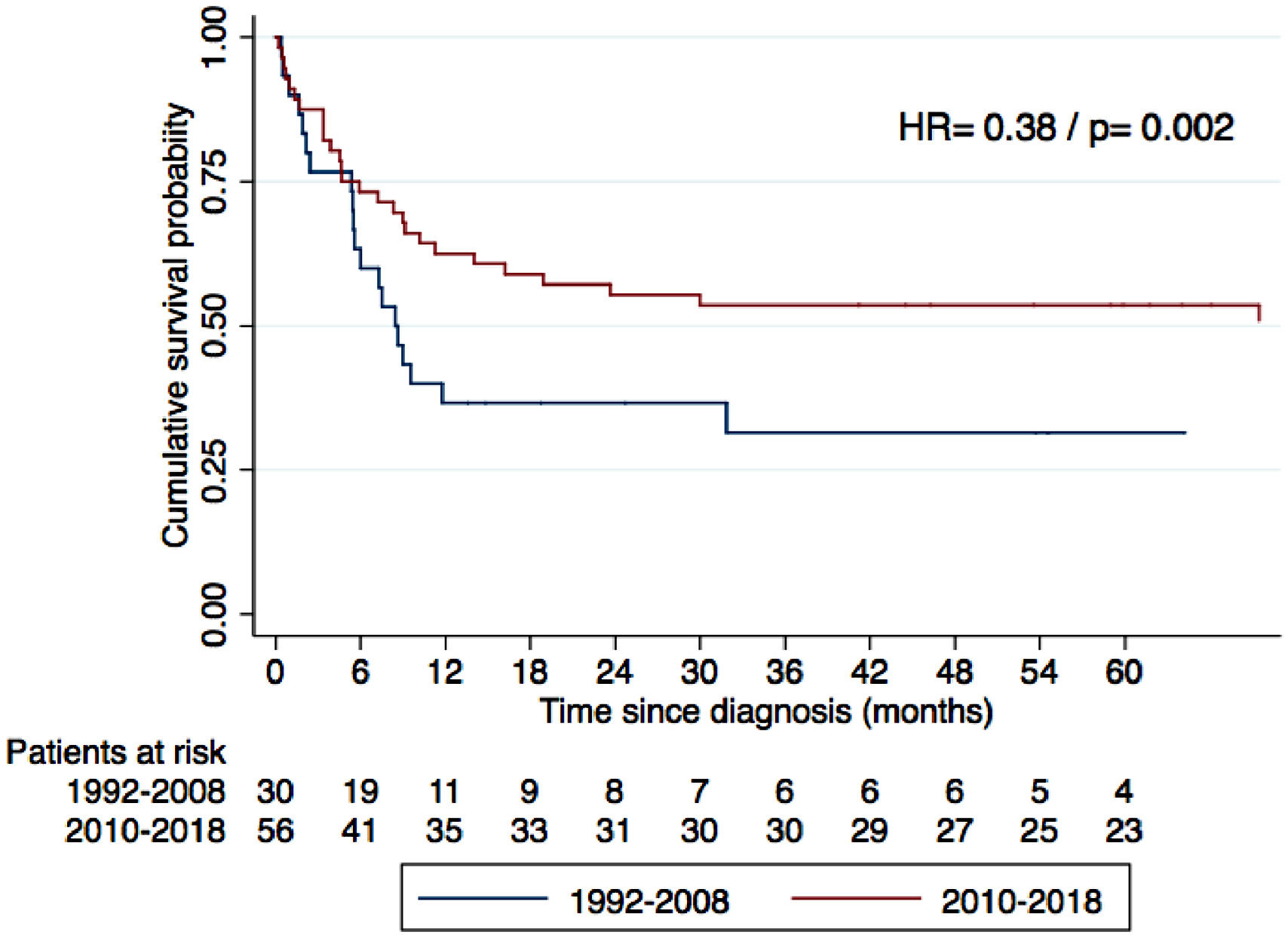
Main ResultsEighty-four patients were included. The most common histological types were Burkitt´s lymphoma (BL), diffuse large B-cell lymphoma (DLBCL), Hodgkin´s lymphoma (HL) and plasmablastic lymphoma (PBL) at 31%, 27%, 21% and 14%, respectively. The three-year OS for the whole cohort of BL, DLBCL, HL and PBL was 58.9%, 65.2%, 47.4%, 76.4% and 50%, respectively. Compared to the cohort of 1992 to 2008, a global increase in the OS was found after excluding HL and adjusting for age and clinical stage (HR 0.38, p = 0.002). However, when the main types were analyzed individually, the increase in the OS was statistically significant only in DLBCL (HR 0.29, p = 0.007). Most patients with DLBCL received CHOP chemotherapy, as in the previous cohort.
ConclusionThe OS has improved in this population, despite no major changes in chemotherapy regimens, mainly due to the universal access to antiretroviral therapy.
During the last decade, Chile has become one of the countries with the highest incidence of the Human Immunodeficiency Virus (HIV) in South America.1 Between 2010 and 2018, new cases almost doubled, reaching 6,948 confirmations in 2018 alone.2 Fortunately, universal access to the combined antiretroviral therapy (cART) and more experienced healthcare teams, among other factors, have improved outcomes for people living with HIV (PLWH) throughout the country.3
In the cART era, this population still suffers from high cancer-related morbidity and mortality, lymphoma being one of the main contributors.4 Even in developed countries, PLWH are still 10 to 20 times more likely to develop lymphoma than the general population.5 The most frequent are aggressive B-cell lymphomas, including the diffuse large B-cell lymphoma (DLBCL), Burkitt's lymphoma (BL) and plasmablastic lymphoma (PBL). Primary central nervous system lymphoma (PCNSL), once being a common acquired immunodeficiency syndrome (AIDS)-defining event, has dramatically reduced its incidence with the advent of the cART. Hodgkin's lymphoma (HL), although not considered an AIDS-defining event, has increased its frequency in the post-cART era.6
International studies addressing AIDS-related lymphomas (ARL) have shown better outcomes during the last decade,7,8 attributed to effective cART regimens, more intensive (immuno)chemotherapy protocols and improvements in supportive care.
In Chile, there are a few published reports regarding ARL.9-12 Among them, it is worth noting the study by Cabrera et al., who evaluated the overall survival (OS) in 55 consecutive patients with ARL diagnosed between 1992 and 2008. For the whole cohort, the three-year OS was only 27%.12
The aim of this study was to describe a current cohort of patients with ARL treated at a Chilean reference public-funded hospital. We hypothesized that the OS had improved in this population after universal access to the cART was fully implemented.
MethodsThis was a retrospective single-center cohort study. Those enrolled were all consecutive patients with lymphoma and HIV infection who had been diagnosed at our institution between January 2010 and December 2017.
The National Cancer Treatment Guidelines13 issued by the Chilean Ministry of Health were followed for the diagnostic work-up and oncological treatment. Only surgical specimens were obtained (core-needle biopsies were not available). The histological classification was performed by a single pathologist, as per the 2008 World Health Organization criteria.14 Borderline cases were discussed by a pathological-hematological committee. The staging was performed using routine laboratory contrast-enhanced Computed Tomography (CT) and bone marrow biopsy. Positron emission tomography-computed tomography (PET-CT) was not available. In patients with neurological symptoms or BL, cerebrospinal fluid (CSF) cytology and/or flow cytometry, was routinely undertaken.
Clinical stages were defined by the Ann Arbor criteria. Stage I was defined as the involvement of a single lymphatic site; or localized involvement of a single extra-lymphatic organ or site in the absence of any lymph node involvement (IE). Stage II was defined as the involvement of two or more lymph node regions on the same side of the diaphragm; or localized involvement of a single extra-lymphatic organ or site in association with regional lymph node involvement with or without involvement of other lymph node regions on the same side of the diaphragm (IIE). Stage III was defined as the involvement of lymph node regions on both sides of the diaphragm. Stage IV was defined as diffuse or disseminated involvement of one or more extra-lymphatic organs, with or without associated lymph node involvement; or isolated extra-lymphatic organ involvement in the absence of adjacent regional lymph node involvement, but in conjunction with disease at distal site(s).
In terms of prognostic assessment, the Hasenclever International Prognostic Score (IPS) was used in HL,15 the modified St Jude risk stratification in BL11 and the International Prognostic Index (IPI) for the remaining non-Hodgkin's lymphoma (NHL).16
The treatment was based on the aforementioned National Guidelines. The ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine)17 regimen was used for all HLs. For patients with BL treated before 2011, the CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone)18 regimen was used. Since 2011, the modified GMALL-B-ALL/NHL2002 (rituximab, vincristine, iphosphamide, methotrexate, etoposide, cytarabine, cyclophosphamide, doxorubicin and dexamethasone)11 regimen was offered to the young and fit and R (rituximab)-CHOEP (rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide and prednisone), to older/frail patients.19 For DLCBL and other types of aggressive B-NHL, CHOP was used until 2017 and then exchanged for R-CHOP.18 For T-NHL, CHOP or CHOEP were used, depending on patient fitness.
Radiotherapy was used as needed based on standard clinical indications, mainly bulky disease or small persistent lesions after chemotherapy. Patients who refused to undertake first-line chemotherapy or considered too frail, were offered less-intensive regimens and/or palliative care.
The HIV serology was part of routine work-up for all lymphoma cases and positive results were confirmed in the national reference laboratory (Instituto de Salud Pública, Santiago). Following the National HIV Guidelines recommendations,20 all patients were offered cART, if not already using it. Since 2004, access to cART in Chile has been guaranteed by law. Depending on the clinical situation, cART was started, continued or modified by the infectious diseases team.
Demographic and clinical information was obtained from medical records. If available, viral loads and CD4 counts were added, up to two months before the lymphoma diagnosis. The cART regimen being used at diagnosis or in the first regimen started after the lymphoma diagnosis was recorded. The CD4 count was measured by flow cytometry. Viral load was determined by the real-time polymerase chain reaction (RT-PCR) and its results are expressed as log 10 copies/ml of plasma.
In patients receiving first-line chemotherapy with curative intent, OS was estimated from diagnosis until death by any cause or October 31, 2020 (end of the follow-up).
To compare the OS across two periods of time, we obtained individual survival data from a previously reported cohort, which included 55 consecutive patients with ARL, diagnosed between 1992 and 2008 at the same center.12 Of these, 35 patients were treated with curative intent. The overall survival was compared globally between treated patients from the two cohorts, excluding HL cases (with known better prognosis) and adjusting for age and lymphoma stage. In addition, the OS was compared between the cohorts for the most frequent histological types, adjusting for age and clinical stage.
Demographic and clinical characteristics at the time of the lymphoma diagnosis were summarized using the median (range) or percent (frequency), as appropriate. The overall survival was estimated using the Kaplan-Meier method. To compare the clinical variables between cohorts, the Mann-Whitney-Wilcoxon and Chi-square tests were used, as appropriate. To compare the OS between cohorts, a multivariate model adjusted for age and lymphoma stage was used, using Cox proportional hazards method. For the statistical analysis, the STATA 13 software was used.
Informed consent was obtained for every patient before starting the treatment and this study was approved by the Institutional Ethics Committee (Comité de Ética Científica del Servicio de Salud Metropolitano Oriente, Santiago, Chile).
ResultsBetween 2010 and 2017, 982 patients with lymphoma were diagnosed, 84 of them (8.5%) being HIV-positive. Clinical characteristics of the cohort are presented in Table 1. The median age was 41 years (ranging from 21 to 78 years) and 91.6% were male. In 40.7% of the patients, lymphoma and HIV infection were diagnosed simultaneously. We were able to obtain the cART data from 55 patients (Table 2). Among the cases with a previous HIV diagnosis, 75% were using cART. Twenty-six percent of the patients presented also with an opportunistic infection.
Clinical characteristics of 84 patients with HIV and lymphoma diagnosed between 2010 and 2017.
| N° (%) | |
|---|---|
| Age | 41 (21–78) |
| Male sex | 77 (91.6%) |
| Concomitant diagnosisa (n = 81) | 33 (40.7%) |
| Active opportunistic infection (n = 73) | 19 (26%) |
| Median CD4 count /ml (n = 68) | 154 (4–866) |
| Median Log viral copies / mL plasma (n = 72) | 3.56 (1 – 6.43) |
| Patients with undetectable viral load (n = 72) | 22 (30.5%) |
| Advanced stage (III + IV) | 70 (83.3%) |
| Bone marrow infiltration (n = 65) | 25 (38.4%) |
| Adverse risk scoreb | 42 (56.7%) |
| Main histological subtypes | |
| Burkitt's Lymphoma | 26 (30.9%) |
| DLBCL | 23 (27.4%) |
| Hodgkin's Lymphoma | 18 (21.4%) |
| Plasmablastic Lymphoma | 12 (14.2%) |
| T/NK Lymphoma | 2 (2.4%) |
| Others | 3 (3.6%) |
| Extranodal involvement in NHL (n = 66) | 33 (50%) |
| Gastrointestinal site | 19 (57.5%) |
IPS: International Prognostic Score; IPI: International Prognostic Index; DLCBL: diffuse large B-cell lymphoma; NK: natural killer; NHL: non-Hodgkin's lymphoma.
cART regimens used for patients with HIV and lymphomaa.
3TC: lamiduvine; ABC: abacavir; ATV: atazanavir; AZT: zidovudine; cART: combined antiretroviral therapy; DRV: darunavir; EFV: efavirenz; FTC: emtricitabine; NVP: nevirapine; LPV: lopinavir; r: ritonavir; RAL: raltegravir.
BL was the most common histological type (30.9%), followed by the DLBCL (27.4%), HL (21.4%) and PBL (14.2%). Eighty-three percent of the patients were diagnosed with advanced stages (III+IV) and 38.4% presented bone marrow infiltration. Half of the NHLs presented extra-nodal involvement, being the gastrointestinal tract the most frequent site (19/33, 57.5%).
Seventy-three patients (86.9%) received first-line (immuno)chemotherapy with curative intent. The distribution of the treatments used is described in Table 3. With a median follow-up of 42 months, the three-year OS for the whole cohort of BL, DLBCL, HL and PBL was 58.9%, 65.2%, 47.4%, 76.4% and 50%, respectively.
Chemotherapy protocols used for patients with HIV and lymphoma, when treated with curative intent (n = 73).
HL: Hodgkin's lymphoma; BL: Burkitt's lymphoma; DLCBL: diffuse large B-cell lymphoma; GMALL: German Multicenter Acute Lymphoblastic Leukemia Group; PL: plasmablastic lymphoma.
Compared to the 1992–2008 cohort, we observed an evident rise in annual cases, and a relative increase in HL, going from 10.9% in the earlier cohort to 21.4% in the latter. In terms of prognosis, a significant increase in the OS was noted when analyzing all the NHL patients treated, after adjusting for age and clinical stage (Figure 1). When comparing the main histological subtypes, all presented increases in OS, but only in DLBCL was statistical significance reached (See supplementary material). In this group, the three-year OS went from 29 to 47.4% after adjusting by age and clinical stage (HR 0.29, p = 0.007). The two cohorts are further compared in Table 4.
Comparison between two cohorts of patients with HIV and lymphoma from the same institution.
| 1992 - 200812 (n = 55) | 2010 - 2017(n = 84) | p-value | |
|---|---|---|---|
| Mean cases / year | 3.2 | 10.5 | < 0.01 |
| Median age (range) | 38 (23–67) | 41 (21–78) | 0.7 |
| Male sex | 81.8% | 91.6% | 0.08 |
| Median CD4/ml count (range) | 108 (2–575) | 154 (4– 866) | 0.18 |
| Concomitant diagnosisa | 43.6% | 39.2% | 0.69 |
| Main histological subtypes | 0.13 | ||
| Burkitt's Lymphoma | 21.8% | 30.9% | |
| DLBCL | 43.6% | 27.4% | |
| Hodgkin's Lymphoma | 10.9% | 21.4% | |
| Plasmablastic Lymphoma | 9.1% | 14.2% | |
| Advanced Stage (III + IV) | 64% | 83.4% | 0.01 |
| Three-year OS in treated patients | 1992–200813(n = 35) | 2010–2017(n = 73) | p-value b |
| Hodgkin's Lymphoma | 50% | 76.5% | 0.237 |
| DLBCL | 29% | 47.4% | 0.007 |
| Burkitt's Lymphoma | 20% | 65.2% | 0.250 |
| Plasmablastic Lymphoma | 50% | 50% | 0.761 |
DLCBL: Diffuse large B-cell lymphoma.
Our study describes the characteristics and outcomes of a current cohort of patients with ARL, treated in real-world conditions in a middle-income country. As is commonly described in the literature, we found mostly young males presenting with advanced disease and a high frequency of extranodal involvement, being bone marrow infiltration and gastric involvement the most frequent sites. Diagnosis at advanced stages is still an unresolved issue, which further impacts survival.7
Notably in our series, BL was the most frequent type, over DLBCL. This could be explained by several reasons. First, our institution is a referral center for BL for most of northern Chile, but not for other lymphomas, selecting the study population. In addition, this cohort presents higher CD4 counts than in the previous period, which can be associated with a lower frequency of DLBCL and a relative increase in HL and BL.21 It must be taken into account that the differentiation among highly proliferative B lymphomas can be difficult, particularly in the immune deficiency setting.22 On the other hand, the high percentage of male patients is explained by the demographics of the HIV infection in Chile, where approximately 85% of the PLWH are men.2,3
Almost half of our patients did not know their HIV status at the moment of the lymphoma confirmation, as seen in other Latin American reports.23 Unfortunately, this situation has not changed over the last decade. This is a priority issue to address in our region. Since serological tests are widely available, an important point to highlight is the attainment of the HIV serology as part of the initial work-up of all lymphoma patients. In most cases, it is feasible to start the cART concurrently with (immuno)chemotherapy, which is associated with better outcomes.24 Exceptions must be made with zidovudine, cobicistat and ritonavir, which are generally avoided due to their excessive toxicity or pharmacological interactions.
It was surprising that 25% of our cohort, with a previously known HIV diagnosis, were not on cART. This might be partially explained not only by the fact that until 2013 the National Guidelines recommended cART only for patients with CD4 counts below 200/mL or AIDS-defining conditions, but also by non-adherence to the treatment. This issue clearly merits further analysis, but unfortunately this data was not available for all the patients.
SurvivalIn the early years of the AIDS epidemic, most patients with ARL could not tolerate chemotherapy and commonly received only palliative care, resulting in a median OS of approximately 4 months.7 The advent of the cART allowed for the use of more aggressive protocols in this population, progressively improving life expectancy. Nevertheless, in real-world scenarios, the OS is still worse than that of the general population with lymphoma.5,7,8,24 Although this issue was not directly addressed in this study, unpublished data from our institution also showed worse outcomes in ARL (Peña et al. XXI Chilean Congress of Hematology, 2018).
This difference in OS from that of the general population, especially noted in DLBCL, has several potential causes. First, patients with HIV tend to present with advanced disease and biologically aggressive tumors; case series have shown an increased proportion of the activated B-cell (ABC) subtype and the EBV-associated DLBCL in PLWH.25–27 Both situations are associated with poor treatment response and shorter survival. In addition, in many low- and middle-income countries, chemotherapy protocols have tended to be less intensive for this population and therefore, less effective. One example worth noting is rituximab, which until a few years ago, was not universally funded for patients with HIV and B-cell lymphomas in Chile. Even though the only phase-3 randomized clinical trial with patients with HIV and B-cell lymphomas did not show a statistically significant difference in progression-free survival or OS,18 several subsequent phase-2 trials showed benefit when this anti-CD20 monoclonal antibody was incorporated into chemotherapy regimens.24 Currently, rituximab containing protocols (e.g., R-CHOP) are considered the standard of care for DLBCL. The aforementioned trials reported an OS ranging from 75% at two years up to 95% at three years in patients without adverse predictors at diagnosis.
Rituximab has also been progressively added to dose-intensive regimens used in BL. In our series, most patients were treated with a modified GMALL-B-ALL/NHL2002 regimen, including the drug, showing similar results than the largest series published so far.28
Hodgkin's lymphoma, despite not being considered an AIDS-defining neoplasia, presents 5 to 20 times more frequently in PLWH.6 The prognosis has improved notably in the cART era, with similar outcomes, compared to the general population.29
Plasmablastic lymphoma continues to be a poor prognosis neoplasia, despite the cART.30 The overall survival found in our CHOP-treated cohort, despite being seemingly encouraging, could be attributed to the scarce number of treated patients (n = 10), precluding further analysis.
Comparison with the previous cohortWhen comparing with the 1992–2008 cohort, the rise in ARL cases is patent, reflecting the climb in PLWH and their longer survival. Although we observed trends towards a better OS in all histological types, this improvement was statistically significant only in DLBCL. We hypothesize this is due to the scarce number of cases, when comparing with the remaining types.
The OS improvement observed in DLBCL deserves further analysis. As already mentioned, no major changes in chemotherapy protocols were observed across the two periods (only three patients received R-CHOP between 2010 and 2018). These results may be better explained by the universal access to the cART in Chile and, possibly, to the more effective treatment of complications and opportunistic infections.
LimitationsOur study has several limitations. As with any retrospective report, missing data in some clinical records represent potential biases. Specifically, we could not obtain all the data regarding the type and dynamics of cART regimens utilized, precluding further analysis on their impact on clinical outcomes. In addition, even after excluding HL cases, cohorts do not have the same subtype distribution, which could affect survival analysis. Finally, being a single-center study, our results do not necessarily represent the national reality.
ConclusionsThe overall survival has improved in patients with ARL treated at our institution, probably because of the universal access to the cART and better general medical care. It is necessary to further assess the impact of rituximab on this population and to compare current outcomes with a similar HIV-negative population. The high frequency of concomitant HIV and advanced lymphoma diagnosis is still an unsolved issue.
The authors would like to thank Dr. Nicolás Silva for proofreading an earlier draft of this article.