The diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) and, despite all the progress in this field, central nervous system infiltration (CNSi) still occurs at an incidence of 2–10%. The objective of the present study was to evaluate the Central Nervous System International Prognostic Index (CNS-IPI) score in daily practice regarding the reproducibility in a heterogeneous cohort apart from a clinical trial.
MethodsPrimary DLBCL patients were eligible for this study, between January 2007 and January 2017. All patients were treated with rituximab-based chemotherapy, mostly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). The CNSi was diagnosed by liquor (positive cytology and/or immunophenotype), computerized tomography, magnetic resonance image and/or fluorodeoxy-glucose-positron emission tomography, requested only in symptomatic patients when the CNSi was clinically suspected. The CNS-IPI was assessed by graphical comparison and calibration.
ResultsAfter applying the inclusion/exclusion criteria, 322 patients were available for the analysis. The median follow-up was 60 months and the median age was 58 years. Seven patients experienced CNSi, characterizing an incidence of 2.17% (7/322). Comparing groups of patients with and without CNSi, we observed that the lactate dehydrogenase (LDH), number of extranodal sites, IPI, kidney/adrenal and absence of complete response were statistically different. The CNS-IPI model stratified patients in a three-risk group model as low-, intermediate- and high-risk. In our cohort, using the same stratification, we obtained an equivalent the 2-year rate of CNS relapse of 0.0%, 0.8% and 13.8%, respectively.
ConclusionOur study reinforces the reproducibility of the CNS-IPI, specifically apart from clinical trials, and suggests the CNS-IPI score as a tool to guide therapy.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), corresponding to one-third of the cases.1 Treatment has improved over the years, with approximately 70% of overall survival (OS) in 5 years.2 Despite all the progress in this field, central nervous system infiltration (CNSi) still occurs, ranging between 2 and 10%.3-5 In spite of the CNSi being a relatively rare event, it is considered a devastating complication, with refractoriness to treatment and death in the majority of cases.6 Thus, the CNSi is an unmet medical need.
Defining high-risk patients who consequently need prophylaxis seems to be the most adequate approach. Different risk models have been described over the years.7-9 It is known that all of them are based on retrospective studies which included clinical and laboratorial findings only, which can always incur in errors. Nevertheless, while more precise risk factors are not daily-practice available, such as genetic aberrations,10 clinical models would still be useful in defining risk and in deciding therapy strategies.
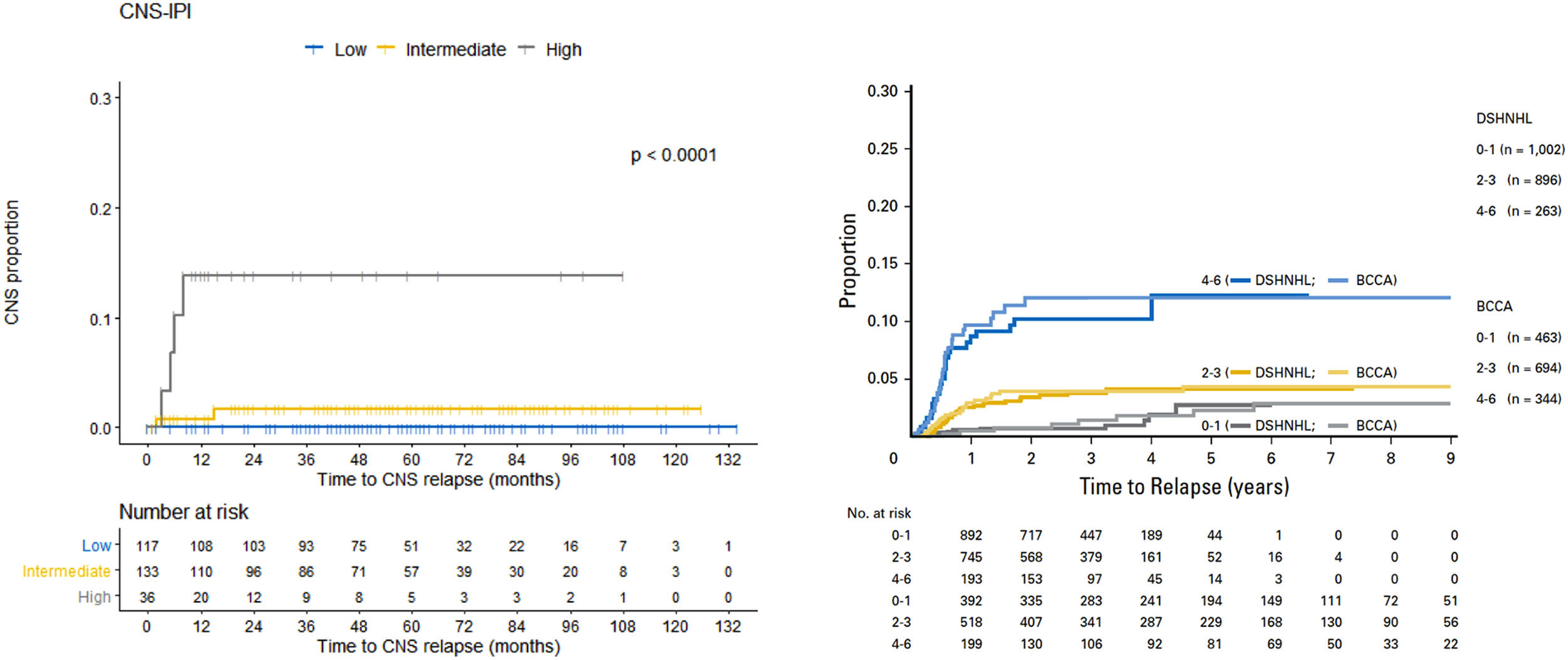
In this manner, the Central Nervous System International Prognostic Index (CNS-IPI) is a robust and well-designed model. This score was developed by the German High-Grade Non-Hodgkin Lymphoma Study Group/MabThera International Trial (DSHNHL/MinT) and validated by the British Columbia Cancer Agency Lymphoid Cancer (BCCA). The CNS-IPI consists of the individual International Prognostic Index (IPI) factors (age > 60; lactate dehydrogenase (LDH) > normal; Eastern Cooperative Oncology Group (ECOG) > 1; Stage III–IV; extranodal involvement > 1) and involvement of kidney and/or adrenal glands, totalizing six risk factors (1 point for each factor). This model stratified patients in a three-risk group model (low- [0–1 point], intermediate- [2–3 points] and high-risk [4–6 points]) and demonstrated 2-year rates of CNS disease of 0.6%, 3.4% and 10.2%, respectively.11
ObjectiveThe objective of the present study was to evaluate the CNS-IPI score in daily practice in a heterogeneous cohort apart from a clinical trial.
MethodologyPatient inclusion and exclusion criteriaThis retrospective research study was approved by the Santa Casa de Misericórdia de São Paulo Medical School (SCMSP) and by the A. C. Camargo Cancer Center (ACCC) Ethics Committees. All participants were studied in accordance with the Helsinki Declaration and the Nuremberg Code, also respecting the Brazilian National Health Council (Resolution CNS 466/2012). Informed consent was signed by all patients who were still in follow-up at both institutions (excluding those lost to follow-up and deceased whose family could not be localized).
As this study included two different institutions, the selection of patients was distinct, so as to have a comparable cohort. At the SCMSP, patients were selected using a pathology base, while at the ACCC, the information system was used to select the C85.9, which is the international disease code, and/or the “diffuse large B-cell”. Patients were deemed eligible for the analysis if they were sequentially diagnosed with primary de novo DLBCL, between January 2007 and January 2015, as well as if they presented the complete chart data and pathological review and had started treatment at the respective institution (SCMSP or ACCC).
Patients were excluded if they had primary mediastinal lymphomas, primary CNS lymphoma and secondary CNS infiltration at diagnosis. They were also excluded if they were less than 18 years old; had human deficiency virus (HIV); were treated without rituximab-based chemotherapy or in cases of early death [< 1 month of follow-up, unless among those started on therapy – mostly postmortem diagnosis (Supplement Figure 1A–C)].
Patient characteristics, treatment and CNS prophylaxisPatients were submitted to physical examination, routine chemistry profiles and image procedures, such as computed tomography (CT), magnetic resonance imaging (MRI) and/or fluorodeoxy-glucose-positron emission tomography (FDG-PET). Lymphoma staging was defined according to the Ann Arbor system12 and patients were also categorized by the International Prognostic Index – IPI13 at baseline. Stage, nodal and extranodal sites had been evaluated on patients charts and/or image (when available) and/or image report from radiology. All patients were treated with rituximab-based chemotherapy, mostly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). Variations observed were R-mini-CHOP and R-CHOP (adapting for tolerance and age). However, considering the retrospective nature, they were not individualized for statistical analysis. Response to chemotherapy followed the Revised International Working Group response criteria.14,15
The CNS prophylaxis included at least one cycle of methotrexate (12–15 mg), plus dexamethasone (4 mg) intrathecal with or without intrathecal cytarabine 40 mg. Intravenous high-dose methotrexate (MTX-HD) at 3.0–3.5 g/m2 was also included.
CNS infiltrationCNS infiltrations were diagnosed by cerebrospinal fluid-CSF (positive cytology and/or immunophenotype), CT, MRI and/or FDG-PET, requested only in symptomatic patients when secondary CNS infiltration was clinically suspected. Confirmed cases were classified as parenchymal, leptomeningeal or both, following the Halderson et al. classification.16
Statistical analysisPatient characteristics were summarized by average, medians and ranges for continuous variables and frequencies and percentages for categorical variables. Patient characteristics were compared between patient groups using the Mann-Whitney test for continuous variables and the Chi-square test/Fisher's exact test for categorical variables.
The Kaplan–Meier method was utilized for survival analysis. The OS was calculated from the date of pathological diagnosis to death, or to the last date of follow-up, while the time to CNS relapse [(TTNS) 1-survival probability] was calculated from the date of pathological diagnosis to CNS relapse, or death, or to the last date of follow-up (the last two were censored) [1-survival probability]. The impact on survival of clinical and therapeutic variables [Kaplan–Meier (1-survival probability)] was evaluated by comparing survival curves by means of the log-rank test.
The difference between groups was determined by univariable analysis using the Chi-square test or Fisher's exact test. Moreover, the differences were analyzed considering time to the event using the Kaplan–Meier method and log-rank test.
The Cox model (Supplement Table 1) was used for analyses of the potential risk factor. The univariate analysis followed what was described by the original CNS-IPI11 (IPI factors individually and kidney/adrenal). The multivariate analysis was not performed.
The CNS‐IPI was assessed by graphical comparison between our results of Kaplan–Meier curves (risk stratification groups: low-, intermediate- and high-risk) and those previously defined by the CNS‐IPI11 and by calibration.
The significance level was fixed at 5% for all tests. Statistical analyses were performed using the IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) and the R software version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
ResultsPatient characteristicsFrom January 2007 to January 2015, 388 patients were diagnosed with de novo DLBCL (nodal and extra nodal) at the SCMSP. After applying the inclusion and exclusion criteria described in the methods section, 151 patients were available for analysis. In the same period, 403 patients were diagnosed with de novo DLBCL (nodal and extra nodal) at the ACCC. Using the same inclusion and exclusion criteria, 171 patients were selected. Therefore, the total number of available DLBCL patients for analysis was 322. Distributions between the two institutions were of 55% at ACCC vs. 46% at the SCMSP. The detailed consort is available in Supplement Figure 1A–C.
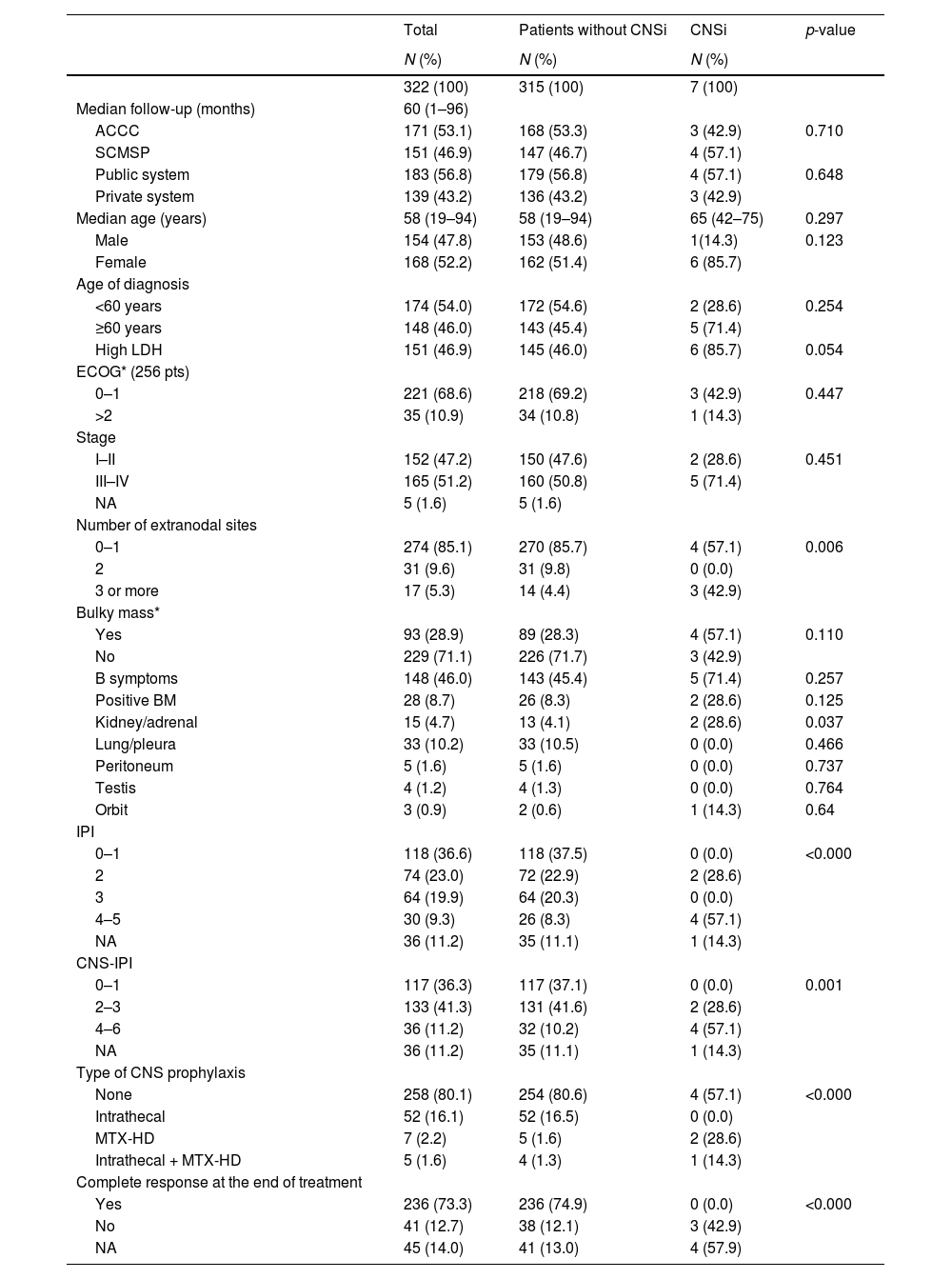
Analyzing the entire cohort and the group of patients with or without CNS infiltrationPatient characteristics are summarized in Table 1. Considering the entire cohort, the median follow-up was 60 months (ranging from 1 to 96 months) and the median age was 58 years. There was a similar distribution between males and females (47.8% vs. 52.2%, respectively) and also a similar distribution between localized – including nodal and extranodal – (stages I–II) and advanced disease (stages III–IV), 47.2% vs. 51.2%, respectively. More than half of the patients had a low or low-intermediate IPI (59.6%) and low and intermediate CNS-IPI 0–3 (77.6%). The minority was submitted to CNS prophylaxis (19.9%), and the majority achieved complete response at the end of therapy (73.3%). The statistics are described in Table 1.
Patient characteristics, including group with and without central nervous system infiltration (statistical analysis).
| Total | Patients without CNSi | CNSi | p-value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| 322 (100) | 315 (100) | 7 (100) | ||
| Median follow-up (months) | 60 (1–96) | |||
| ACCC | 171 (53.1) | 168 (53.3) | 3 (42.9) | 0.710 |
| SCMSP | 151 (46.9) | 147 (46.7) | 4 (57.1) | |
| Public system | 183 (56.8) | 179 (56.8) | 4 (57.1) | 0.648 |
| Private system | 139 (43.2) | 136 (43.2) | 3 (42.9) | |
| Median age (years) | 58 (19–94) | 58 (19–94) | 65 (42–75) | 0.297 |
| Male | 154 (47.8) | 153 (48.6) | 1(14.3) | 0.123 |
| Female | 168 (52.2) | 162 (51.4) | 6 (85.7) | |
| Age of diagnosis | ||||
| <60 years | 174 (54.0) | 172 (54.6) | 2 (28.6) | 0.254 |
| ≥60 years | 148 (46.0) | 143 (45.4) | 5 (71.4) | |
| High LDH | 151 (46.9) | 145 (46.0) | 6 (85.7) | 0.054 |
| ECOG* (256 pts) | ||||
| 0–1 | 221 (68.6) | 218 (69.2) | 3 (42.9) | 0.447 |
| >2 | 35 (10.9) | 34 (10.8) | 1 (14.3) | |
| Stage | ||||
| I–II | 152 (47.2) | 150 (47.6) | 2 (28.6) | 0.451 |
| III–IV | 165 (51.2) | 160 (50.8) | 5 (71.4) | |
| NA | 5 (1.6) | 5 (1.6) | ||
| Number of extranodal sites | ||||
| 0–1 | 274 (85.1) | 270 (85.7) | 4 (57.1) | 0.006 |
| 2 | 31 (9.6) | 31 (9.8) | 0 (0.0) | |
| 3 or more | 17 (5.3) | 14 (4.4) | 3 (42.9) | |
| Bulky mass* | ||||
| Yes | 93 (28.9) | 89 (28.3) | 4 (57.1) | 0.110 |
| No | 229 (71.1) | 226 (71.7) | 3 (42.9) | |
| B symptoms | 148 (46.0) | 143 (45.4) | 5 (71.4) | 0.257 |
| Positive BM | 28 (8.7) | 26 (8.3) | 2 (28.6) | 0.125 |
| Kidney/adrenal | 15 (4.7) | 13 (4.1) | 2 (28.6) | 0.037 |
| Lung/pleura | 33 (10.2) | 33 (10.5) | 0 (0.0) | 0.466 |
| Peritoneum | 5 (1.6) | 5 (1.6) | 0 (0.0) | 0.737 |
| Testis | 4 (1.2) | 4 (1.3) | 0 (0.0) | 0.764 |
| Orbit | 3 (0.9) | 2 (0.6) | 1 (14.3) | 0.64 |
| IPI | ||||
| 0–1 | 118 (36.6) | 118 (37.5) | 0 (0.0) | <0.000 |
| 2 | 74 (23.0) | 72 (22.9) | 2 (28.6) | |
| 3 | 64 (19.9) | 64 (20.3) | 0 (0.0) | |
| 4–5 | 30 (9.3) | 26 (8.3) | 4 (57.1) | |
| NA | 36 (11.2) | 35 (11.1) | 1 (14.3) | |
| CNS-IPI | ||||
| 0–1 | 117 (36.3) | 117 (37.1) | 0 (0.0) | 0.001 |
| 2–3 | 133 (41.3) | 131 (41.6) | 2 (28.6) | |
| 4–6 | 36 (11.2) | 32 (10.2) | 4 (57.1) | |
| NA | 36 (11.2) | 35 (11.1) | 1 (14.3) | |
| Type of CNS prophylaxis | ||||
| None | 258 (80.1) | 254 (80.6) | 4 (57.1) | <0.000 |
| Intrathecal | 52 (16.1) | 52 (16.5) | 0 (0.0) | |
| MTX-HD | 7 (2.2) | 5 (1.6) | 2 (28.6) | |
| Intrathecal + MTX-HD | 5 (1.6) | 4 (1.3) | 1 (14.3) | |
| Complete response at the end of treatment | ||||
| Yes | 236 (73.3) | 236 (74.9) | 0 (0.0) | <0.000 |
| No | 41 (12.7) | 38 (12.1) | 3 (42.9) | |
| NA | 45 (14.0) | 41 (13.0) | 4 (57.9) |
ACCC: A. C. Camargo Cancer Center; SCMSP: Santa Casa Medical School São Paulo; LDH: lactate dehydrogenase; ECOG: eastern cooperative oncology group; NA: not available; BM: bone marrow; IPI: International Prognostic Index; CNS-IPI: Central Nervous System International Prognostic Index; CNS: central nervous system; MTX-HD: high-dose methotrexate.
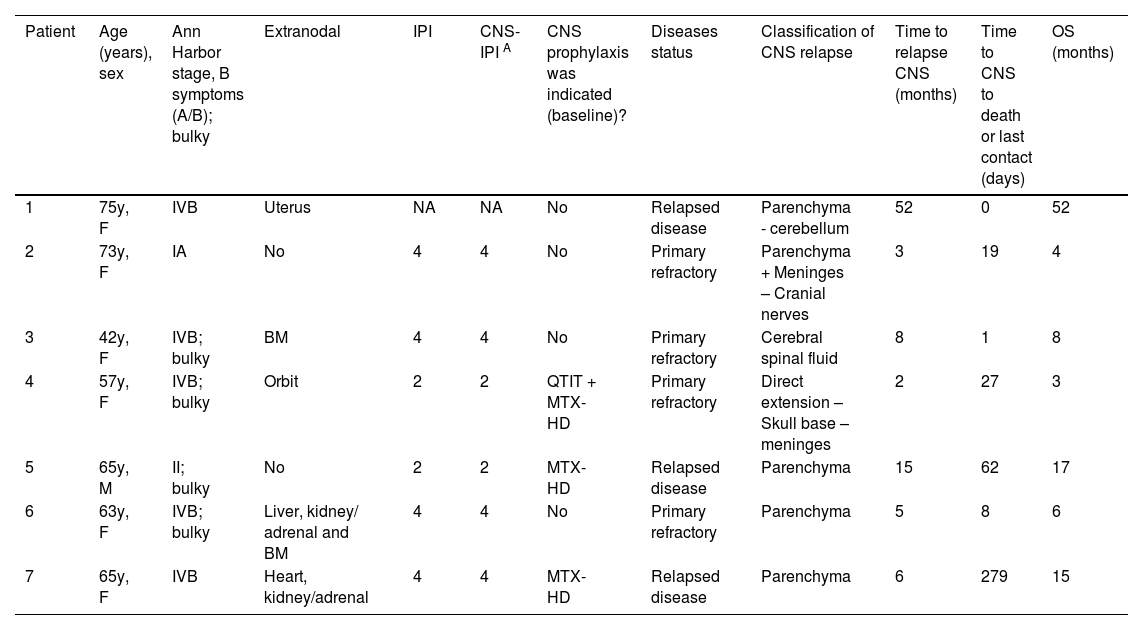
A total of 7 patients experienced CNSi (7/322). The median time from diagnosis of DLBCL to CNSi was six months (range 2–52 months), while median time of CNSi to death was 19 days (range 0 days–9.3 months). Four patients presented with brain parenchymal involvement, two patients presented with leptomeningeal disease and one patient presented with both. The CNS-IPI was retrospectively calculated for these patients (Table 2).
Central nervous system infiltration time and characteristics.
CNS-IPI: Central Nervous System-International Prognostic Index; CNS-IPIA: CNS-IPI retrospectively calculated; CNS: central nervous system; OS: overall survival; F: female; M: male; BM: bone marrow; QTIT: intrathecal chemotherapy; MTX-HD: high-dose methotrexate.
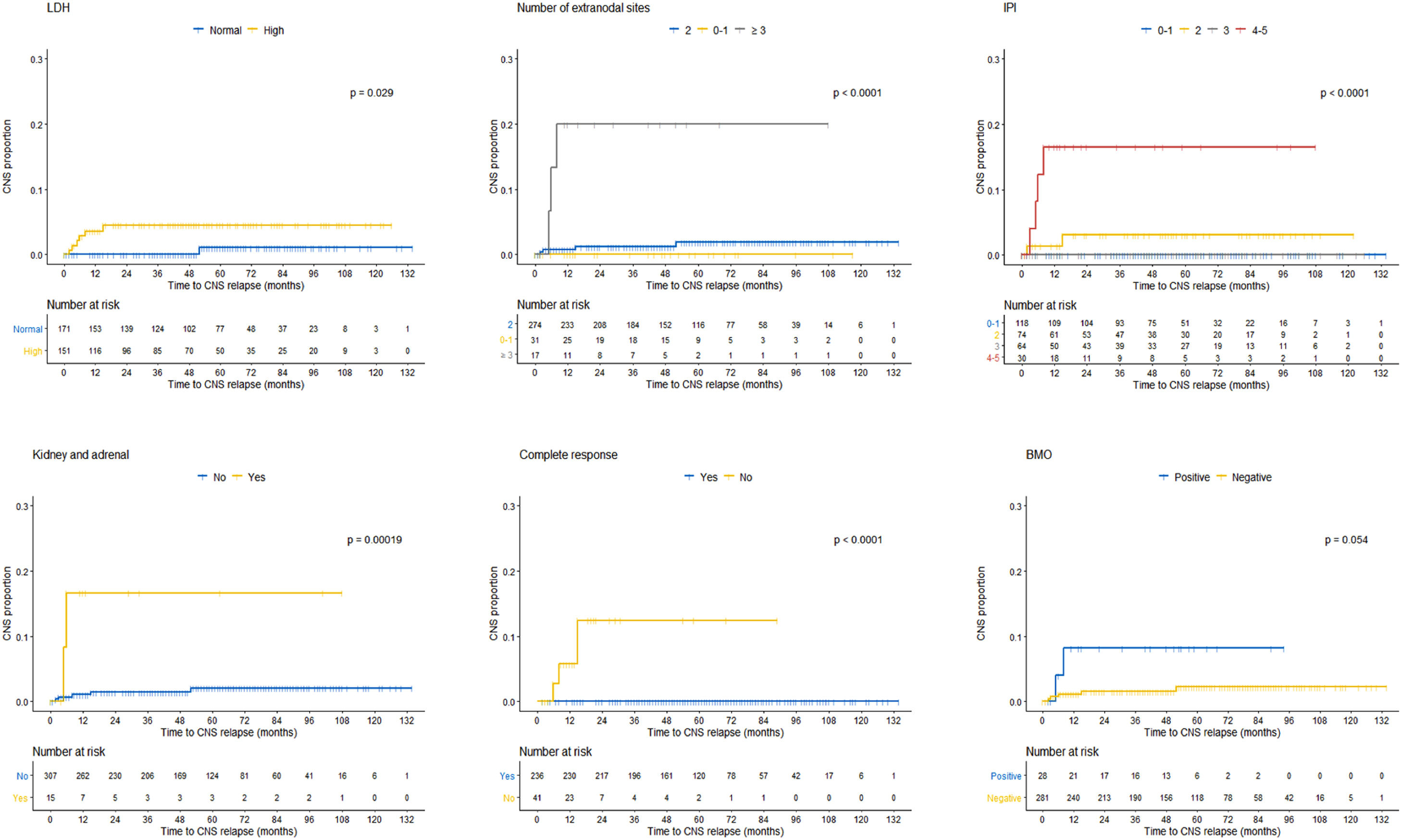
As previously described, comparison between groups demonstrated at the baseline that the LDH, number of extranodal sites, IPI, kidney/adrenal were statistically different in patients without and with CNSi (Table 1). Furthermore, regarding the treatment, the absence of complete response was also statistically different. Differences between patients without and with CNSi were also analyzed considering time to the event (Kaplan–Meier curves), with similar results, except for positive bone marrow (BM) biopsy (p = 0.059) (Figure 1).
A univariate analysis model was also performed in our cohort. Nonetheless, a multivariable analysis was not possible, given the small number of events/relapses (7/322) and the existence of 6 predictors in the original model (age > 60; LDH > normal; ECOG > 1; Stage III–IV; extranodal involvement > 1; kidney and/or adrenal glands) – Supplement Table 1.
A demonstrative table comparing our univariate and multivariate results from the CNS-IPI model (including original model and validation cohort) can be seen in Supplement Table 2.
CNS-IPI scoreThe CNS-IPI was retrospectively calculated in 286/322 patients. These 36 missing data were mainly due to an inadequately performed ECOG performance and staging. All CNS-IPI evaluations described above were based on 286 patients.
We observed that most of the characteristics of the cohort without CNSi were comparable or even equivalent to the entire cohort. However, there was a statistical difference between patients with and without CNSi in some specific characteristics. Patients with CNSi had a higher IPI, 4–5 (57.1% vs. 8.3%, p < 0.000), were more frequently in the high-risk CNS-IPI group (57.1% vs. 10.2%, p = 0.001) and most of them did not achieve complete response at the end of therapy (42.9% vs. 12.1%, p < 0.000). In addition, patients with CNSi had more frequently 3 or more extranodal sites (42.9% vs. 4.4%, p = 0.006) and specific sites, such as adrenal/kidney involvement (28.6% vs. 4.1%, p = 0.037).
The original CNS-IPI model stratified patients in a three-risk group model, low- [0–1 point], intermediate- [2–3 points] and high-risk [4–6 points]) and demonstrated a 2-year rate of CNS relapse of 0.6%, 3.4% and 10.2%, respectively. In our cohort, we respected the same stratification: low-, intermediate- and high-risk. Similarly, the 2-year rate of CNS relapse was of 0.0%, 0.8% and 13.8%, respectively (Figure 2).
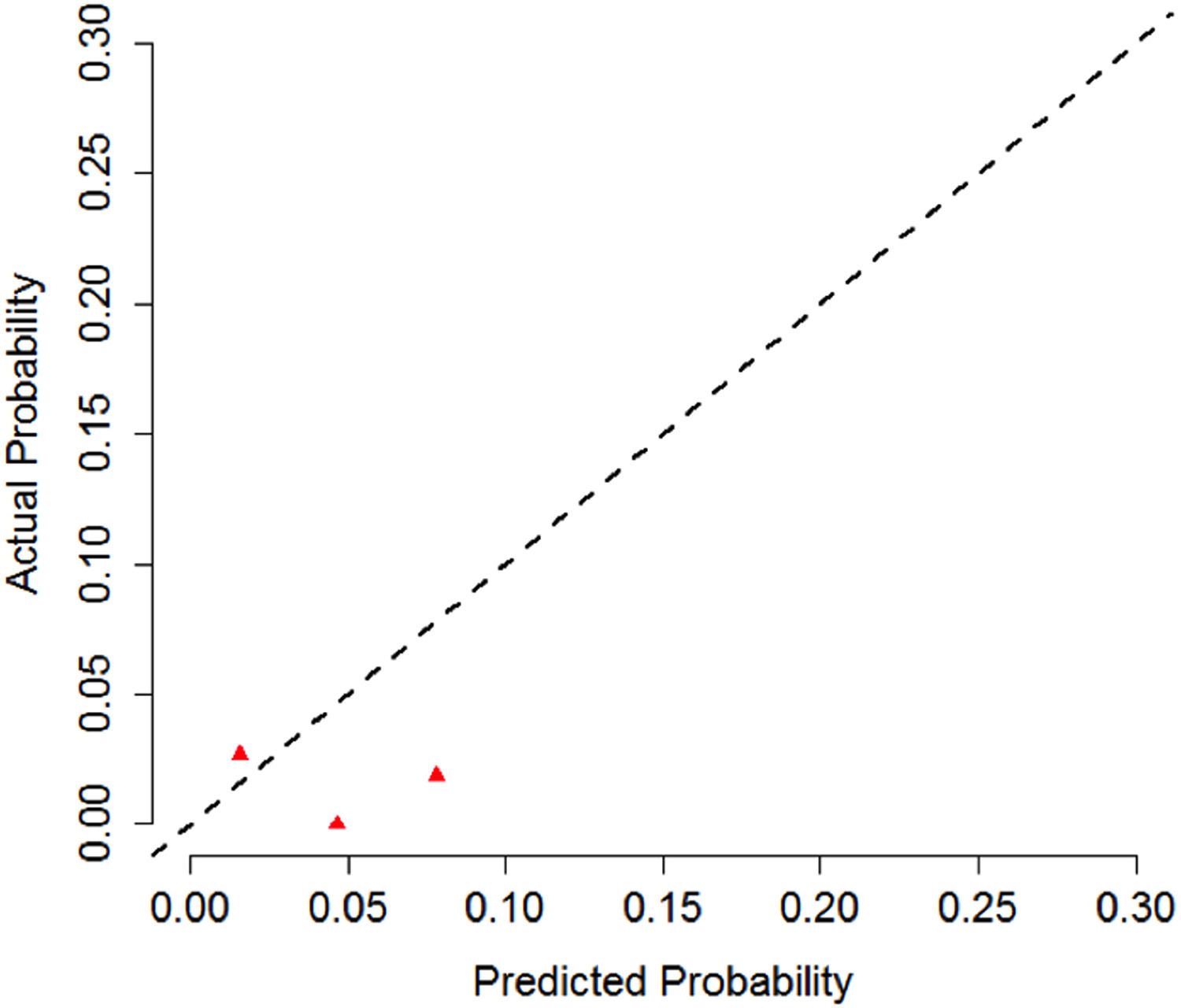
Calibration of the CNS-IPI model was also performed. First, we calculated the CNSi probability in 2 years for the entire cohort (CNS-IPI original model). Then, we compared the estimated probability with the events observed in our cohort (real probability). Based on the results, a calibration curve was built. This analysis, nevertheless, is limited by the small number of events (7 patients with CNSi) in Figure 3.
CNS prophylaxisThe majority of patients without CNSi were not submitted to any modality of CNS prophylaxis (80.5%), while 3 patients with CNSi were submitted to some modality of CNS prophylaxis (MTX-HD- 2 patients; Intrathecal + MTX-HD - 1 patient), p < 0.000; Table 1).
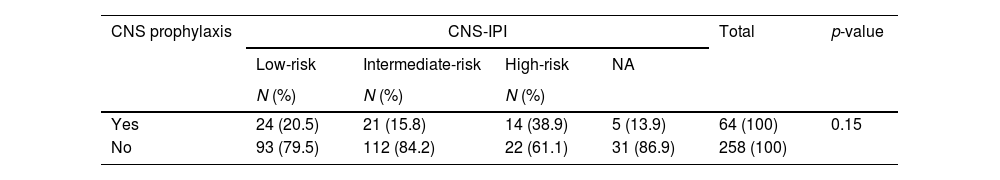
The CNS prophylaxis was administered according to a physician's decision, due to the inexistence of an institutional protocol at SCMSP and at ACCC. We sought to understand the rationale to perform CNS prophylaxis by calculating the CNS-IPI retrospectively (Table 3). We observed that the majority of patients considered high-risk CNS-IPI were not submitted to prophylaxis (61.1% vs. 13.9%, p = 0.015).
CNS-IPI retrospectively calculated in patients submitted to CNS prophylaxis.
CNS: central nervous system; CNS-IPI: Central Nervous System-International Prognostic Index; NA: not available.
Comparison between the types of CNS prophylaxis applied would be inadequate in our study, considering there were only 7 events (2.17% incidence of CNSi).
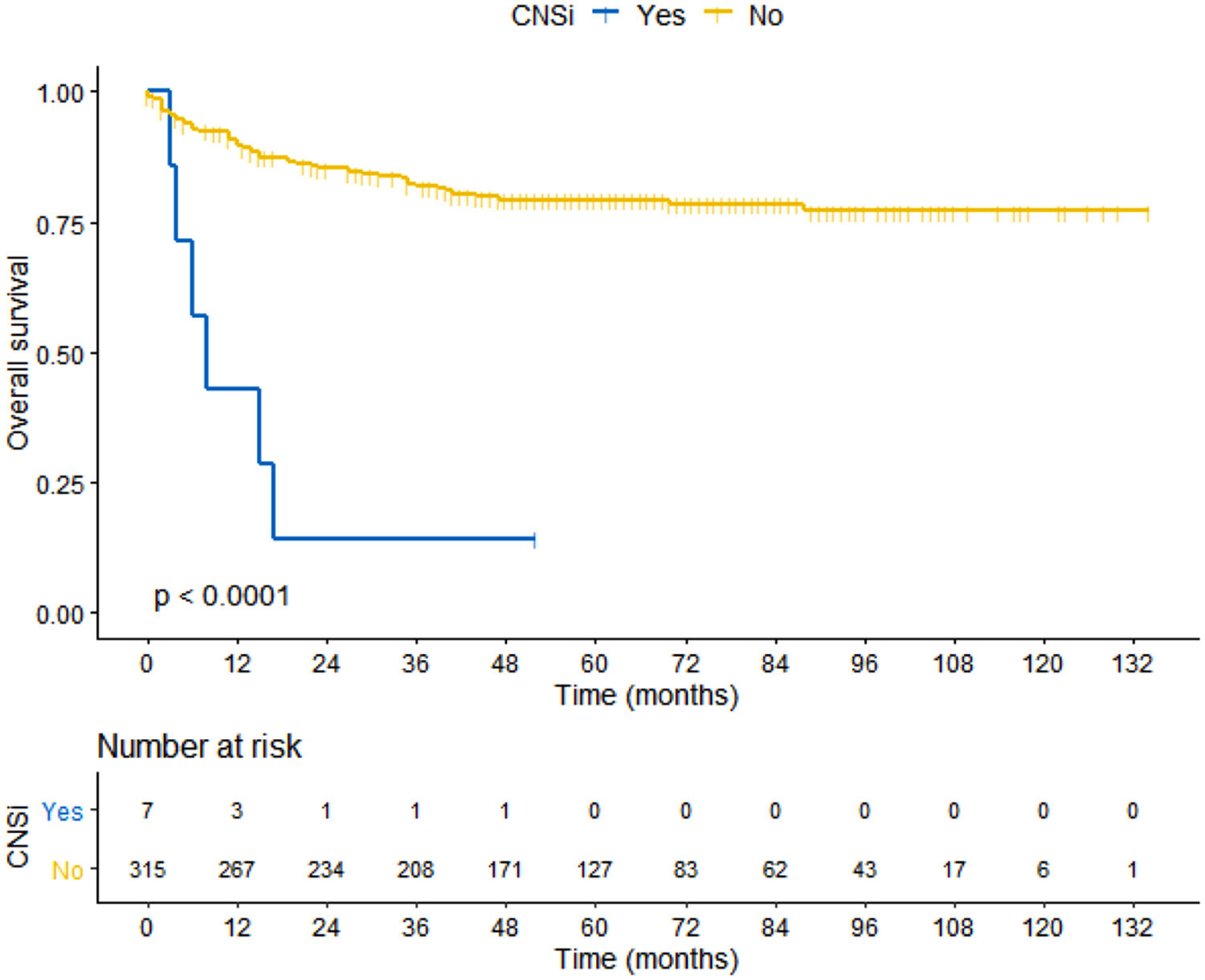
SurvivalThe median OS for the entire cohort was not reached and was estimated at 88% in 5 years (average: 8.8 years [95% CI: 8.3–9.3]). OS in ACCC cohort was estimated at 86% in 5 years (average: 8.6 years [95% I: 8.1–9.1]) and OS in the SCMSP cohort was estimated at 74% in 5 years (average: 8.1 years [95% CI: 7.3–8.9]). There was a statistical survival difference between the cohorts of patients without or with CNSi. The median OS for patients without CNSi was not reached (average: 9.0 years [95%CI: 8.5–9.5]), while the median OS for patient with CNSi was 8 months (95% CI: 2.8–13.1), log-rank p < 0.000 (Figure 4).
DiscussionThe CNSi continues to occur despite excellent results in the first-line treatment of DLBCL. Recent studies, with the incorporation of new monoclonal antibodies (polatuzumab-vedotin) to the backbone CHOP,17 have shown excellent results, with 2-year progression-free survival reaching 70%.17,18 However, the CNSi has remained with its incidence relatively unchanged, with a high rate of morbidity and mortality. In this study, the CNSi rate was 2.17% and the median OS was 8 months, according to literature data.11,19-21
The best way to treat CNSi is through early diagnosis, with treatment intensification/modification, with drugs that cross the blood-brain barrier.11 This is reinforced by better survival results, which occur in patients who infiltrate the CNS at diagnosis.3 Therefore, in clinical practice, prognostic scores are important tools to assess risk, early diagnosis and to define therapeutic management.11,18
Subclassing the population along the lines of the lymphogene classification10 and ctDNA22,23 cerebrospinal fluid seems to be the way to best treat patients with DLBCL. This has been the scope of most clinical studies on DLBCL, but they are still far from being used in clinical practice. In this sense, scores such as the IPI18 and CNS-IPI,11 even based on retrospective clinical and laboratory data, are still indispensable.
In our cohort, the CNS-IPI adequately stratified patients, the 2-year rate of CNS relapse, compared to the original CNS-IPI model, was, respectively 0.0% vs. 0.6% for low-risk11; 0.8% vs. 3.4% for intermediate-risk, and; 13.8% vs. 10.2% for high-risk (Figure 2). With the same objective of the present study, the CNS-IPI has also been validated in a large Asian dataset, in which it was considered an adequate tool for risk stratification.24
To build the CNS-IPI score, a risk factor model has been cautiously proposed by the DSHNHL/MInT and this was also performed with the BCCA cohort. However, in our cohort, given the small number of events (seven cases of CNSi), producing a risk factor model was a limitation. Only the kidney/adrenal were able to predict risk in our univariate analysis. Even so, we statistically compared the groups of patients without and with CNSi and observed differences in the following characteristics: LDH, number of extranodal sites, IPI, kidney/adrenal, BM and orbit. Respecting the different methodology, it presented a result similar to the risk factors proposed by the original study, reinforcing the importance of these baseline characteristics for the CNSi (Table 1).
Our data reinforce the potential of the IPI (Figure 1) to also stratify high-risk patients, but the inclusion of the kidney and adrenal as extranodal sites guarantees the greater specificity. It is an extranodal site which is an isolated predictor of the CNSi, as observed in our univariate analysis (Table 1) and highlighted by Schmitz et al. 20. In clinical practice, this should be an important indicator for CNS prophylaxis.
It is important to emphasize that the number of extranodal sites,7 breast,25 uterus26 and testis27 are also isolated predictors of CNSi (previous works indicate CNSi rates which can reach 30% in testicular lymphoma27) and should not be disregarded, even with low IPI CNS results. Traditionally, they are not included in risk models because of their rarity and specificity, but they should always be considered as risk factors and the patients, as candidates for prophylaxis.
Testicles were not a predictor of risk for CNSi in the DSHNHL/MInT but were considered in the validation of the BCCA. In addition to the rarity, intensive protocols of intrathecal chemotherapy, radiotherapy of the contralateral testicle performed by the German group may also explain the lower incidence of CNSi.27
Other groups are still trying to study other characteristics to increase the power of the CNS-IPI, such as cell of origin (COO) and BCL2 and MYC expression.8,28 Others are studying isolated characteristics which can predict CNSi, such as the number of extranodal sites >2.7 In our study, the number of extranodal sites ≥ 3 detected by CT and/or PET-CT was also a different characteristic between patients without and with CNSi (p < 0.000) (Figure 1).
In high-risk patients, there is an indication for CNS evaluation (through MRI/ CT of the skull and cerebrospinal fluid) and in performing CNS prophylaxis. There is no benefit of intrathecal chemotherapy for DLBCL, which has been previously proven.29
Our data showed that the majority of patients without CNSi were not submitted to any modality of CNS prophylaxis (80.5%). In the meantime, less than half of patients with CNSi were submitted to some modality of CNS prophylaxis (MTX-HD: 28.6%; intrathecal + MTX-HD: 14.3%). Considering the small number of events (7/322), the impact of each specific CNS prophylaxis cannot be analyzed. However, the MTX-HD at the end of treatment still seems to be an effective form of prophylaxis 3,4,29 Its benefit, although questioned, is still recommended.
It is also worth emphasizing that CNS prophylaxis with MTX should not be performed interspersed with RCHOP due to the risk of delaying a notably curative therapy (RCHOP).29 Furthermore, the risk of renal dysfunction can reach up to 9% of cases30 so the indication should be individualized and the risks discussed with the patient.
Treatments, such as R-CHOP + IBTK20,21 or Lenalidomide,22 seem to be the way forward for high-risk patients and may in the future replace CNS prophylaxis, but so far the recommendation of MTX-HD remains (especially in CNS-IPI high-risk patients), in addition to isolated involvement of the kidney, adrenal, testis and breast.
Our analysis is limited by its retrospective design. A similar shortcoming is true of others previously published analyses. The retrospective analysis might result in bias of patient selection, confounding factors and missing data. More studies are still necessary in the field.
ConclusionThe CNSi in DLBCL remains a rare but devastating complication. Despite the limitation of all prognostic indexes, they are of value to stratify patients and to standardize practice. Our study reinforces the reproducibility of the CNS-IPI in daily practice (apart from clinical trials). It reinforces that almost 80% of patients are at low or intermediate risk of CNSi with no need of interventions. Those considered high-risk would benefit from more intensive diagnosis and from the prophylaxis approach. Therefore, it is important to emphasize that the CNS-IPI score is a tool to guide therapy, but it is still necessary to personalize therapy.
This study was funded by subsidy from the Coordination for the Improvement of Higher Education Personnel (CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).