High-dose methotrexate (HDMTX) is an essential part of chemotherapy regimens for hematologic neoplasms. The incidence of acute kidney injury (AKI) after HDMTX in unmonitored outpatient infusion had not been reported in adults yet. In this study, we evaluated toxicity data after outpatient administration of HDMTX without drug monitoring.
MethodsPatients 16 years old or over with acute lymphoblastic leukemia and non-Hodgkin lymphoma who received at least one outpatient infusion of HDMTX without drug level monitoring were included. This is a retrospective, nested case-control study, in which the cases comprised patients who developed AKI after HDMTX.
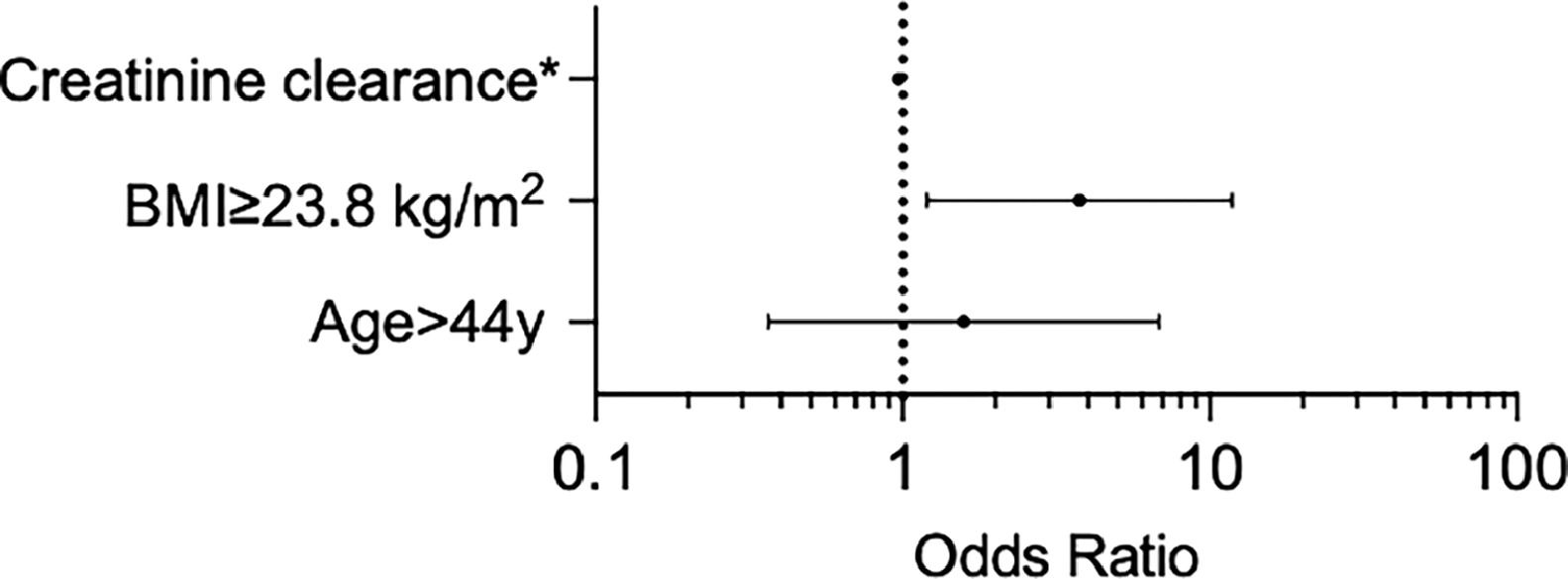
ResultsOverall, 302 patients were included, encompassing 840 infusions. Hospitalization occurred in 8.6 %. A total of 25 patients presented AKI after HDMTX administration, corresponding to 3 % of the methotrexate (MTX) infusions and 8.3 % of the patients. HIV-associated Burkitt lymphoma was more common in patients who presented AKI (18 vs. 6.8 %, p = 0.03). Baseline factors related to AKI after HDMTX were age > 44 y, body surface area ≥ 1.76 m2, body mass index (BMI) ≥ 23.8 kg/m2, glomerular filtration rate, and thrombocytopenia (< 150×109/L). Multivariable analysis for adjusting such factors found that BMI was independently related to AKI after HDMTX (OR 3.8). Death after AKI occurred in 56 %.
ConclusionOur data showed a similar rate of AKI after HDMTX to that reported in the literature, even without drug monitoring. However, patients who developed AKI in our cohort fared worse than expected, with more hospitalizations and death. A higher BMI was associated with the MTX-induced AKI in our cohort, suggesting a differential drug clearance and the need for specific guidelines for obese patients.
Methotrexate (MTX) is an antifolate that has been an essential part of chemotherapy regimens for acute lymphoblastic leukemia (ALL) and specific subtypes of non-Hodgkin lymphoma (NHL), when it is used as high-dose methotrexate (HDMTX, ≥ 500 mg/m2) to overcome the blood-brain barrier.1 These doses are potentially lethal without administering intravenous or oral folinic acid to rescue normal cells from apoptosis, specifically in the bone marrow and gastrointestinal tract.2 Renal injury is arguably the major toxicity of HDMTX, caused by the precipitation of MTX and its metabolites in the renal tubular lumens.3 Acute kidney injury (AKI) induces critical organ damage and death if not detected early and treated, especially in older patients.3 Therefore, these regimens are usually administered in the hospital, with aggressive intravenous hydration, drug level monitoring, urinary alkalinization and MTX level-guided leucovorin rescue.1
Although some factors for drug-induced AKI have been raised and recommendations have been published, the reality is that in resource-poor settings, HDMTX is usually administered in an outpatient clinic and without drug monitoring.1,4,5 Prior reports, especially for short infusions or pediatric populations, have shown that it is feasible and safe for selected patients.6,7
The incidence of AKI after HDMTX in unmonitored outpatient infusion had not been reported in adults yet, since most reports used drug-level monitoring and inpatient infusions. Therefore, in this study, we evaluated our toxicity data after outpatient administration of HDMTX without drug monitoring, aiming to find risk factors for such complications and to describe their outcomes in this real-life setting.
MethodsStudy designThis study was approved by the institutional review board (Certificate number: 61311522.4.0000.0068, https://plataformabrasil.saude.gov.br). This is a retrospective, nested case-control study conducted at the cancer institute (ICESP) of Hospital das Clínicas at the University of Sao Paulo, a large academic medical center located in Sao Paulo, Brazil. On average, more than 5000 patients per month are currently undergoing chemotherapy at ICESP, only in the public health system.8 Study data were collected from electronic medical records after the Institutional Review Board (IRB) approval.9
Patients and treatmentPatients 16 years old and over with ALL and NHL who received at least one outpatient infusion of HDMTX without drug level monitoring between Jan/2010 and Jun/2020 were included in the cohort (see flowchart in supplementary appendix). Most patients with ALL received a 6-hour infusion, in accordance with our local protocol, while those with NHL received a 2-hour infusion in a day clinic.5,6 All patients received intravenous hydration with alkaline solution from day 1, along with oral sodium bicarbonate every 6 h targeting urinary pH ≥ 7. In the chemotherapy room, patients had their urine pH monitored by dipstick. Empirical leucovorin rescue with oral folinic acid was prescribed, starting 24 h from the infusion for 3 days. The folinic acid was administered in a single intravenous 50 mg dose, followed by 30 mg per oral route, every 6 h for 3 days. Patients were followed as outpatients by a multidisciplinary team of experienced doctors and nurses. Some patients received inpatient HDMTX infusion, based on their clinical status and baseline disease, and were excluded from this analysis (n = 73).
Data collection and statistical analysisThe cases were those patients from the original cohort who developed AKI by the Kidney Disease Improving Outcomes (KDIGO) definition (increase in serum creatinine by 0.3 mg/dL or more within 48 h or increase in creatinine level to 1.5 times baseline or more within the last 7 days)10 or with delayed MTX clearance (serum MTX level > 0.1 mM after 72 h from drug infusion)1 when clinically suspected. A nested cohort of controls was composed of patients who did not develop the complication, sampling from the cohort of patients who received HDMTX. Cases and controls were randomly matched 1:2 for the employed protocol only. The estimated glomerular filtration rate (eGFR) was estimated by the Cockcroft-Gault equation.
Pairwise comparisons between patient subgroups were performed by the Mann-Whitney or Kruskal-Wallis tests for continuous variables and by the Pearson's chi-square or Fisher's exact test for categorical variables. Proportions were reported with their respective confidence intervals, when appropriate. Hematologic and liver toxicity were graded according to the Common Terminology Criteria for Adverse Events Version 5.0 (CTCAE v5.0). The conditional logistic regression was used to find baseline risk factors between cases and controls. Missing data were not imputed. All tests were two-tailed with a significant p-value < 0.05.
ResultsA total of 404 patients received at least one HDMTX infusion within the study period, with 102 being excluded (see flowchart in supplementary appendix). Among the 302 patients included, 840 infusions were performed, with a median of 2 infusions per patient, ranging 1 to 10. Most patients were diagnosed with diffuse large B-cell lymphoma (DLBCL) (37 %), Burkitt lymphoma (BL) (19.5 %), or ALL (18 %), followed by other NHL subtypes (25.5 %). Most subjects received HDMTX within an even course of cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride (Adriamycin), methotrexate, cytarabine and the steroid hormone dexamethasone (Hyper-CVAD, or HCVAD), or as single therapy (61 %).
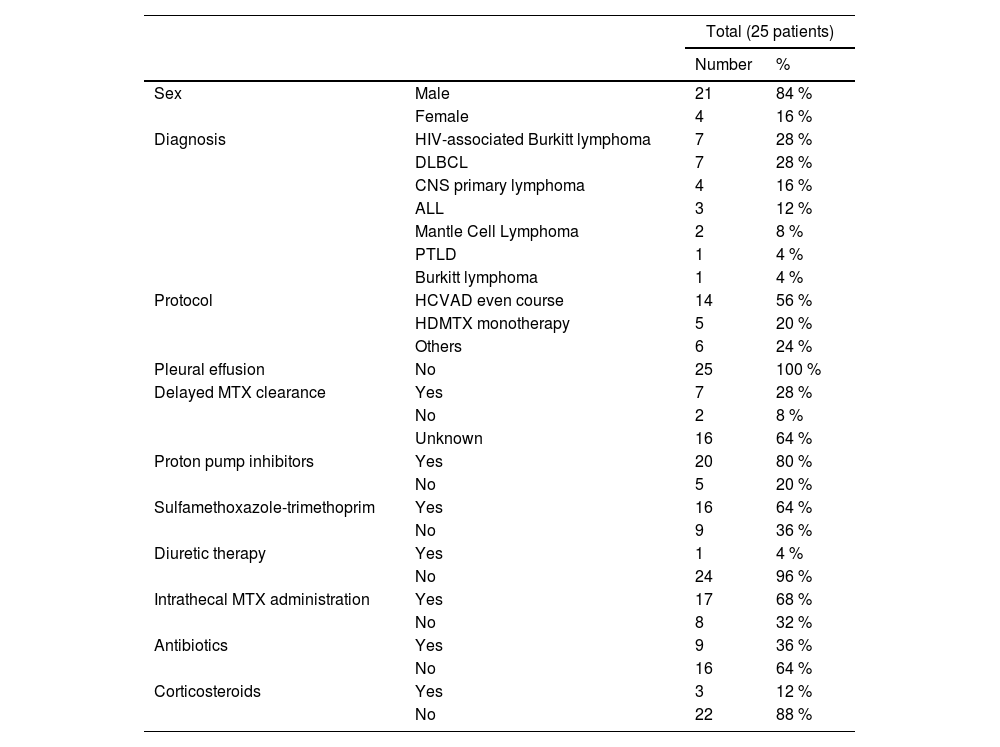
Overall, hospitalization occurred in 8.6 % (73/840) of infusions during the MTX nadir, with 4.6 % (39/840) needing intensive care support, being febrile neutropenia the most common complication (63 %). Among all infusions analyzed, 25 patients presented AKI after HDMTX administration, corresponding to 3 % (95 %CI 2 - 4.4) of MTX infusions and 8.3 % (95 %CI 5.5 - 12.1) of patients. Those patients had a median age of 44 years (range, 20 - 78) and received a median dosage of MTX of 1000 mg/m2 (range, 1000 – 3500). HIV-associated BL was more common in patients who presented AKI vs. those who did not develop this complication (18 vs. 6.8 %, p = 0.03). Remaining baseline characteristics of AKI cases are summarized in Table 1. These identified cases were then matched 1:2 with controls, as previously described.
Characteristics of patients who developed AKI after HDMTX (cases).
Univariate analysis to find pre-treatment factors related to AKI after HDMTX were age > 44 years (OR 3.2 [95 %CI 1 - 9.7], p = 0.045), body surface area ≥ 1.76 m2 (OR 3.4 [95 %CI 1.2 - 9.9], p = 0.039), body mass index (BMI) ≥ 23.8 kg/m2 (OR 3.5 [95 %CI 1.2 - 9.9], p = 0.02), eGFR (OR 0.96 [95 %CI 0.93 - 0.99], p = 0.012), and thrombocytopenia (< 150×109/L) (OR 4.7 [95 %CI 1.7 - 13.1], p = 0.002). A table with all tested variables can be seen in the supplementary appendix. Multivariable analysis for adjusting such factors found that the BMI was independently related to the AKI after HDMTX (OR 3.8 [95 %CI 1.2 - 11.8], see Figure 1).
Among those patients with AKI, only 9/25 had their MTX serum level measured at the event, with delayed MTX clearance in 7/9. Thrombocytopenia, neutropenia and hyperbilirubinemia grade 3 or higher were found in 36 %, 64 % and 36 %, respectively. Among these, 22/25 needed hospitalization, with infection diagnosed in 23/25 and the need for dialysis in 5/25 cases. Death after the AKI eventually occurred in 56 % (14/25), with infection being the immediate cause of death on the medical charts. Most patients presented a positive blood culture during this complication (64 %). The most commonly retrieved agents were Escherichia coli (7/14), Klebsiella pneumoniae (3/14), Candida sp. (2/14) and Acinetobacter sp. (2/14).
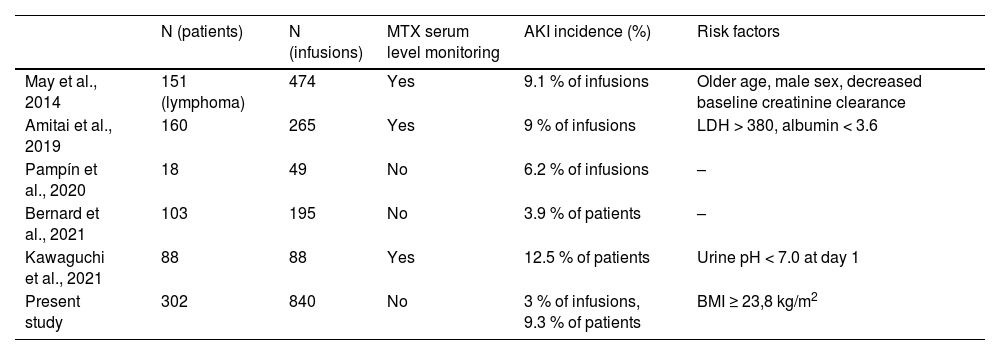
DiscussionIn this study, we extensively reviewed medical charts of patients with leukemia and lymphoma who received HDMTX in the outpatient ward, without drug level monitoring in a 10-year period. The rate of AKI in this cohort was similar to others reported in the literature, regardless of the MTX serum level monitoring (Table 2). In a report by Bernard et al., a 4 % AKI incidence was described in a selected cohort of patients with aggressive lymphoma based on six criteria (age, albumin, performance status, renal and hepatic functions, good understanding and no weight loss) who were receiving outpatient MTX infusion. These patients received outpatient HDMTX with no drug-level monitoring and were clinically monitored. Interestingly, in the mentioned study, only grade I/II AKI were detected, emphasizing proper patient selection's role in the undertaking of such an approach.6 Regarding our study, the treating physician performed the indication of outpatient administration, with a minority of subjects receiving inpatient infusions due to toxicity concerns.
Selected studies with outpatient HDMTX infusion in hematological malignancies.
Although this approach is widely performed, especially in under-resourced settings, few reports address its risks and toxicity. Our analysis showed that this strategy is safe for most patients, with an acceptable toxicity rate. By avoiding hospitalization, this strategy significantly reduces treatment costs, besides improving the patients’ quality-of-life.11–13 The prior literature has also pointed out that patients with hematologic malignancies seem to experience increased HDMTX toxicity, when compared to other cancers.14
Most authors have agreed that a decreased eGFR is a strong risk factor for post-HDMTX AKI.1,15,16 Our study also found its relevance in the univariate analysis. Other studies have also pointed to lactate dehydrogenase, albumin and bilirubin levels as possible factors of this complication.15,16 In our case-control study, age, BMI, eGFR, and baseline thrombocytopenia were all statistically significant for the AKI. Ultimately, the BMI was strongly correlated with the AKI, highlighting that more studies on the MTX pharmacokinetics are needed, especially in overweight individuals. This variable was also tested in studies with fewer patients and no correlation with the AKI was found.15,17 A pivotal study by the St. Jude Total Therapy group showed that intracellular levels of MTX polyglutamates did not differ according to the BMI in children, reinforcing that chemotherapy doses in the protocols must be based on actual body weight, with no adjustment for abnormal body composition.18,19 In a study by Pai et al., aiming to find a model for the estimation of MTX clearance, age, albumin, serum creatinine and vertebral body height emerged as the final variables, highlighting that the kidney function estimation might be the key, instead of the amount of adiposity itself.20
In this cohort, few patients who presented with AKI had their MTX level measured, displaying the low awareness of clinicians regarding this complication at our center. Additionally, our AKI cases were more severe, with high rates of dialysis and infection/death. This might be related to this study's retrospective nature and design, in which only more severe events were captured.
This study has notable limitations regarding its retrospective design and the small number of events within the cohort, restricting the power of statistical analysis. Furthermore, the use of other concomitant drugs could not be comprehensively addressed, although their role is not established in adults.21 Nevertheless, this study reviewed a considerable number of MTXHD infusions and reinforced that our approach is safe for most patients.
ConclusionsOur data showed a similar rate of AKI after HDMTX to that reported in the literature, even without drug monitoring. However, patients who developed AKI in our cohort fared worse than expected, with more hospitalizations and deaths. A higher BMI was associated with MTX-induced AKI in our cohort, suggesting a differential drug clearance and the need for specific guidelines for obese patients. Careful selection of patients for this procedure is warranted.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.