The expression pattern of CD27 and CD44 was found to correlate with the differentiation stages of B cell precursors, which were known to be involved in a variety of immunological responses.
Aim of the studyThis study aimed to determine the biological significance of CD27 and CD44 expression in patients with B-precursor acute lymphoblastic leukemia (B-ALL), as well as their association with standard prognostic factors and therapeutic response.
Patients and methodsThis case-control study included 60 pediatric patients newly diagnosed with B-ALL and 30 pediatric controls. The patient CD27 and CD44 levels were measured using the Beckman Coulter Navios Flow Cytometer.
ResultsMost malignant cells (91.6 %) expressed CD44, but only 50 % of the patients had CD27 expressed. The positive CD 44 expression was associated with unfavorable prognostic markers, including a decrease
The B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is considered the most prevalent type of cancer in children, accounting for approximately 80 % of pediatric leukemia and 20 % of adult leukemisa.1 The age-adjusted incidence rate in the USA is 1.38 per 100,000 individuals per year, with an estimated 5930 new cases and 1500 deaths in 2019.2 At the National Cancer Institute (NCI), Cairo University, the annual incidence of ALL is approximately four cases per 100,000 Egyptian children per year, accounting for 20 % of childhood malignancies.3
The prognosis of BCP-ALL is favorable, as survival rates for pediatric patients have improved over the past years.4 However, specific subgroups continue to have poor outcomes, including patients with specific disease-related cytogenetic and molecular abnormalities.5 Therefore, the identification of new prognostic indicators for patients with poor prognoses is crucial, as it could aid in the development of new therapeutic targets for the treatment of BCP-ALL with a high risk of progression.6
There was a correlation between B-cell precursor differentiation and the expression of two molecules known to play a role in numerous immunological responses. These two molecules are the glycoproteins CD27 and CD44. The CD27 is a member of the (TNFRSF7) tumor necrosis factor (TNF) receptor superfamily. The CD27 is expressed on the surface of T-cells, natural killer (NK) cells, antibody-secreting plasma and memory B cells. The CD27 is considered a general marker for memory B cells.7 In addition, it is crucial for lymphoid differentiation and apoptosis.8 The natural ligand of the CD27 is the CD70, which is expressed only on the activated immune cells.9
On the surface of malignant B-cells in diffuse large B-cell lymphoma, anaplastic large cell lymphoma, peripheral T-cell lymphoma and cutaneous T-cell lymphoma, the CD70 and CD27 are co-expressed.10-11 In patients with ALL, elevated CD27 expression at the time of diagnosis has been associated with a lower-risk classification and a favorable prognosis.12 In contrast, other studies have found that the CD27 is highly expressed in pediatric and adult BCP-ALL, with a high risk of progression, suggesting that this molecule may serve as a therapeutic target.13
The CD44 is a family of non-kinase trans-membrane glycoproteins that are widely expressed on hematopoietic cells, bind to hyaluronic acid and other extracellular matrix components and aid in the homing of hematopoietic cells to their niche.14 In addition to its function as an adhesion molecule, the CD44 can also transduce anti-apoptotic and proliferation signals, which have been implicated in tumor cell proliferation, adhesion, migration and invasion.15
The CD44 has been demonstrated to be a surface biomarker of cancer stem cells (CSCs) and to play a crucial role in tumor initiation and progression. An elevated CD44 expression was correlated with poor prognosis in numerous types of cancer, including lung cancer,16 ovarian cancer,17 hepatocellular carcinoma18 and papillary thyroid carcinoma.19 The CD44 overexpression was associated with a poor prognosis in both T-ALL and acute myeloid leukemia.20 In B-ALL, the CD44 expression was observed in the medium and high-risk patients, suggesting a possible association between this CD44 expression and unfavorable outcomes.21 Therefore, the CD44 may be considered a promising therapeutic target.22
This study aimed to investigate the CD27 and CD44 expression in a cohort of B-ALL cases in order to determine their association with different stages of B-ALL development, maturational subtypes, genetic alterations, clinical characteristics and prognostic significance of these differentiation markers in B-ALL patients.
Patients and methodsEthical approvalThe Institutional Review Board of the NCI, Cairo University, Egypt, approved the study. The ethical approval number was CP2065–30,886, issued on September 11, 2018. All patients, the control group and their guardians gave written informed consent. The study was performed according to the Helsinki Declaration for studies on humans.
PatientsThis case-control study included 60 pediatric patients newly diagnosed with B-ALL and 30 pediatric controls. All patients were recruited from the pediatric outpatient clinic at the NCI, Cairo University, between December 2018 and December 2021. The control group consisted of healthy donors recruited from the Bone Marrow Transplantation Unit. Prior to initiating treatment, bone marrow (BM) samples were obtained from the B-ALL patients. Data on the patient age, gender, diagnosis, treatment and disease status were retrieved from patient files. Patients underwent at least a 24-month follow-up from therapy and were evaluated for complications, such as resistance or relapse.
All cases were submitted to a complete clinical examination investigating the complete blood count (CBC), BM aspirate (BMA) and immunophenotyping (IPT) to specify ALL subtypes, in addition to the DNA index, cytogenetic analysis by conventional karyotyping and fluorescent in situ hybridization, and screening for recurrent cytogenetic ALL abnormalities, such as the t(9;22)(BCR-ABL), t(1;19)(TCF3/PBX1), t(4;11)(mixed lineage leukemia (MLL) gene) and t(12;21) (TEL/AML1) by the conventional polymerase chain reaction (PCR). Samples were collected in EDTA vacutainers at diagnosis (prior to initiating treatment).
Samples were collected at D14, D28 and D42 to monitor the minimum residual disease (MRD), depending on the availability of samples and the presence of the leukemia-associated immunophenotype (LAIP). All patients were treated according to the modified St. Jude total XV protocol (total XV protocol). It includes three phases: induction of remission, consolidation and maintenance.
Flow cytometric analysisThe IPT was performed on either peripheral blood (if the percentage of blasts exceeded 20 %) or bone marrow samples. An acute leukemia panel was applied for all cases. The CD27 (T16) phycoerythrin (PE) and CD44 (HP211) fluorescence isothiocyanate (FITC) were all purchased from Beckman Coulter (Miami, USA). The blast population was gated for analysis by using the CD45 expression versus side scatter. Flow cytometric two-parameter dot plots and quadrant statistics were generated. More than a 20 % reactivity with monoclonal antibodies was considered positive. The samples were analyzed using the Beckman Coulter Navios Flow Cytometer (Miami, USA).
Statistical methodsThe data were analyzed using the GraphPad Prism statistical package (version 6.0f). The numerical data were expressed as mean, standard deviation, median and range, as appropriate. The qualitative data were expressed as frequency and percentage. The Chi-square test (Fisher's exact test) was utilized to assess the relationship between qualitative variables. The Mann-Whitney test (non-parametric t-test) was used to compare two groups for non-normally distributed quantitative data. The ANOVA test with multiple variants was used to compare more than two quantitative groups. The overall survival (OS), disease-free survival (DFS) and event-free survival (EFS) were estimated using the Kaplan-Meier method. The EFS was calculated from the date of diagnosis to the date of progression (relapse or death, whichever happened first) and the OS, until death from any cause. Censored patients included live patients for the OS analysis. For the EFS, those who did not relapse, progress, or die, were considered censored at the last assessment date. A p-value less than 0.05 was considered significant.
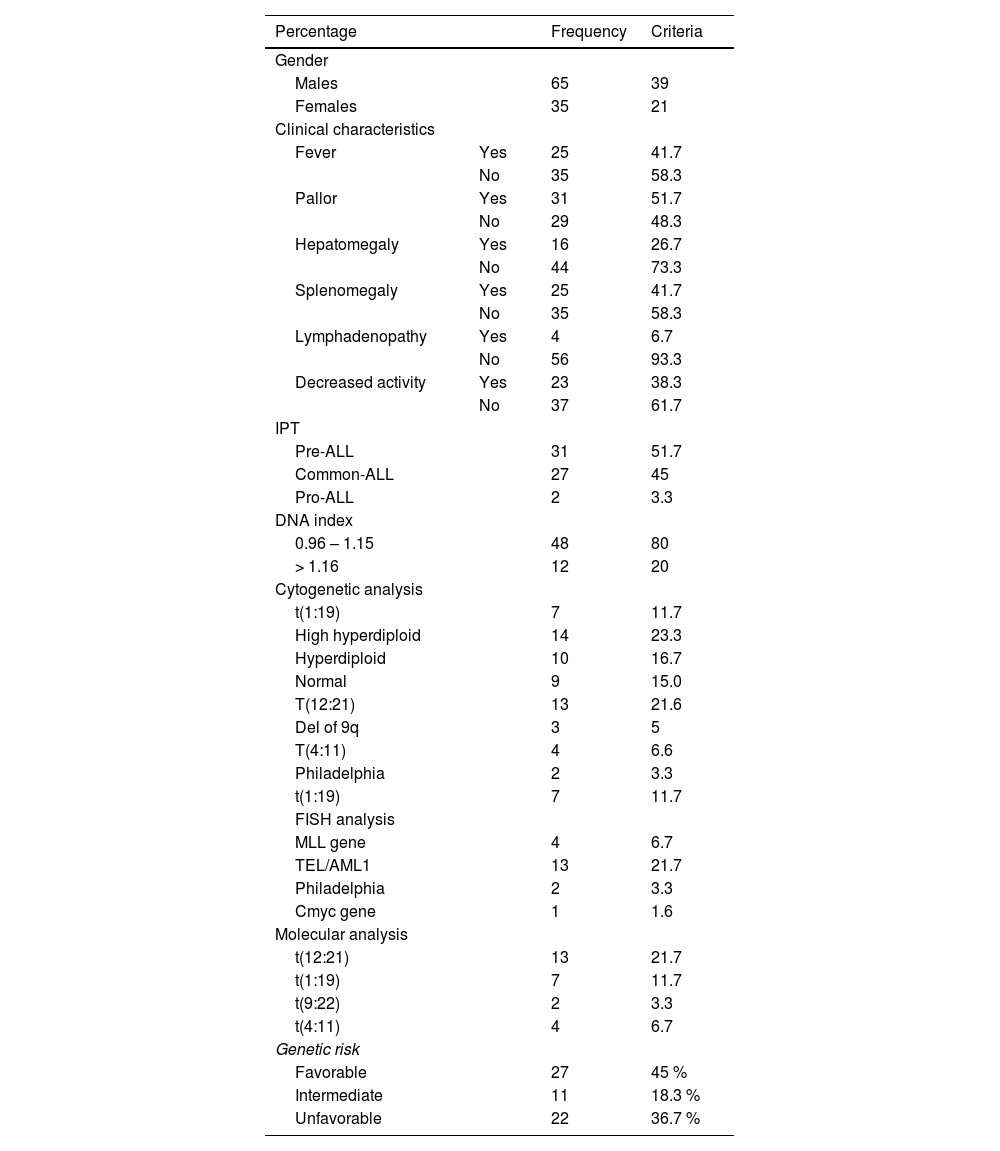
ResultsThe age of our patients ranged from 6 months to 18 years, with a median of 4.25 years and a mean value of 5.95 ± 4.13. The male-to-female ratio was 1.86:1, with 39 males (65 %) and 21 females (35 %). The clinical and laboratory features of the studied ALL patients are displayed in.Table 1. The hematological data of our patients are depicted in Supplementary Table 1. According to cytogenetic abnormalities, patients were classified as favorable, intermediate, or unfavorable.23 (Supplementary Table 2).
Clinical and laboratory features of the studied ALL patients.
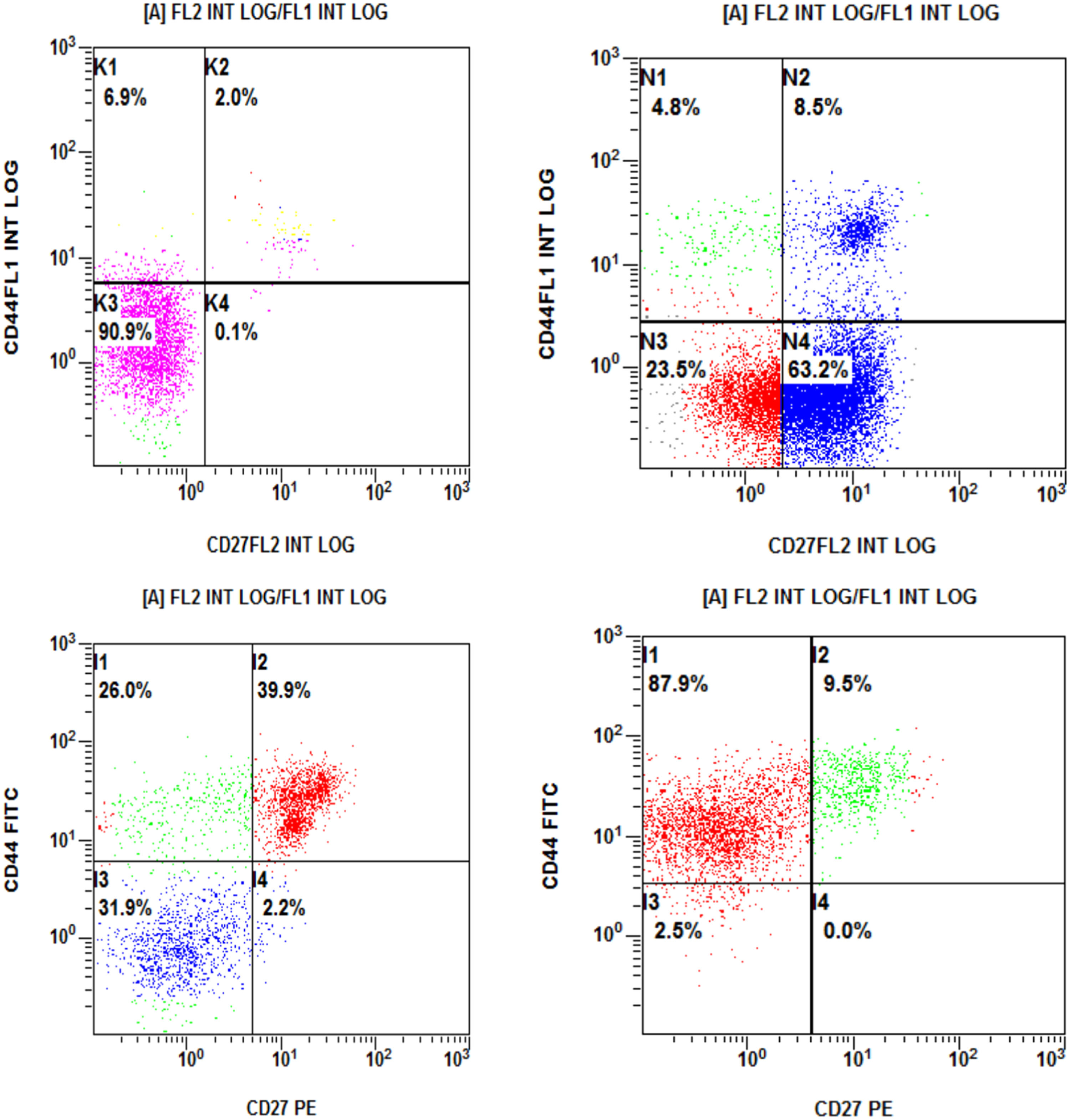
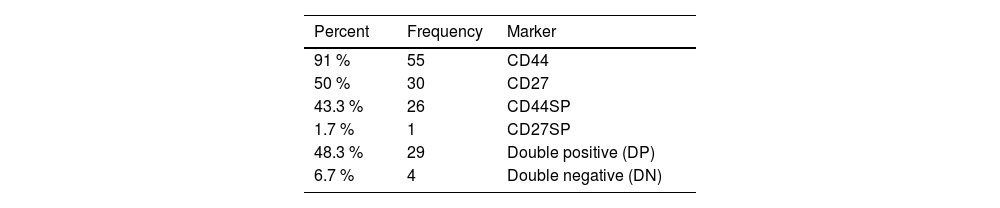
The CD44 was positive in 55 patients (91.6 %) and the CD27, in 30 patients (50 %). A large proportion of cells (91.6 %) express the CD44, with a mean expression of 61.5 %. A relative expression was observed in normal cells, with the CD27 being expressed in 50 % of the patients, with a mean expression of 47.8, but it was expressed in a few normal cells and its primary expression was 14.9 (Supplementary Table 3).
Four patterns of the CD44 and CD27 molecule expression were identified in the CD44 alone [single positive (SP)], with more than 20 % positivity for the CD44 and less than that for the CD27 in 26 patients (43.3 %). The CD27SP showed more than 20 % positivity for the CD27 and less than that for the CD44 in 1 patient (1.7 %), while 29 patients (48.3 %) [double positive (DP)] presented with more than 20 % positivity for both the CD44 and CD27. Four patients (6.7 %) were double negative (DN), with less than 20% positivity for both the CD44 and CD27 molecules (Figure 1,.Table 2).
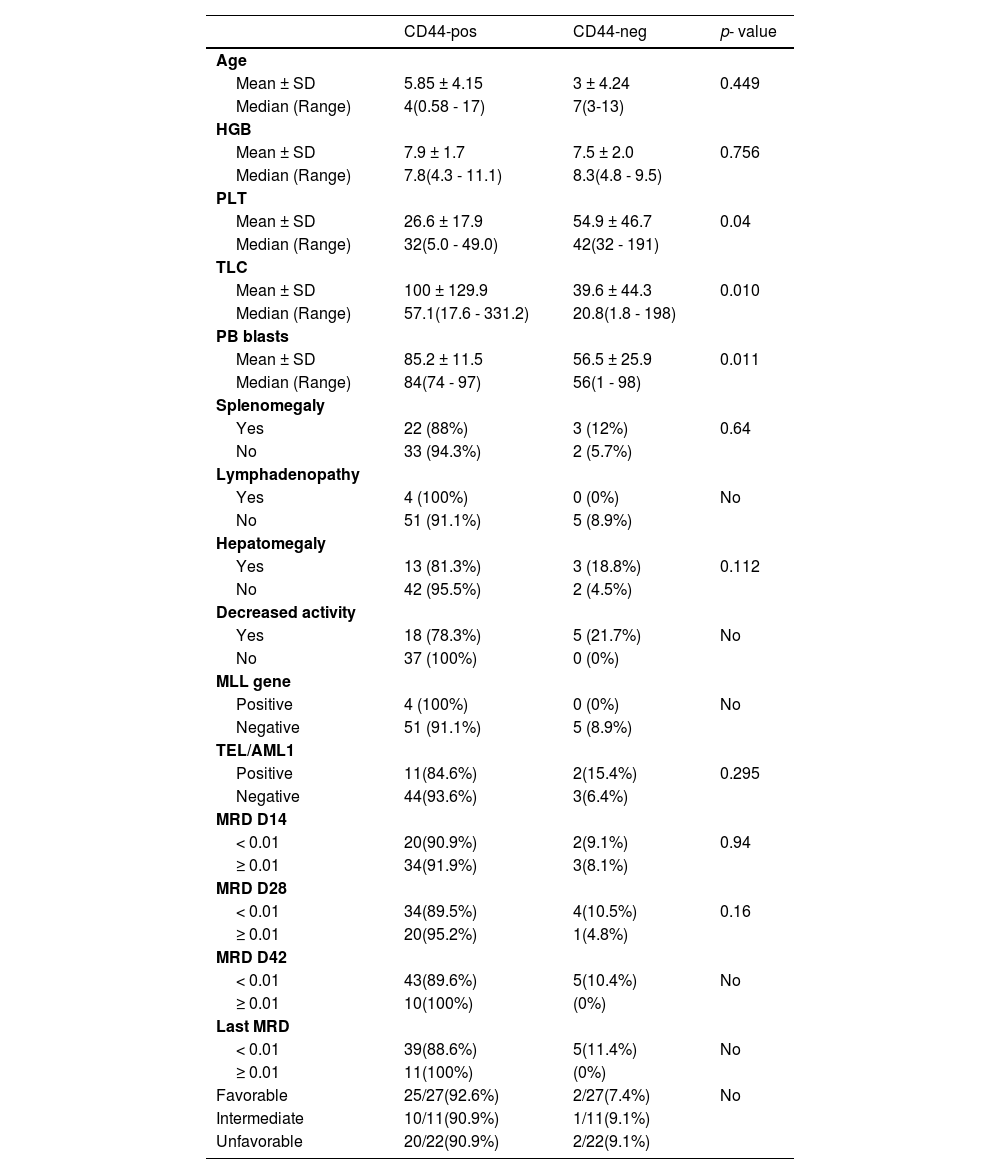
The platelet count was significantly different between CD44-positive and CD44-negative patients, with CD44-positive patients having a lower platelet count (p = 0.04). Patients with CD44 positivity have higher PB blasts than patients with CD44 negativity (p = 0.011). Table 3 shows that the white blood cell (WBC) count was significantly higher in the CD44-positive patients (100 ± 129.9109/L) than in the CD44-negative patients (39.6 ± 44.3 109/L) (p = 0.010) (Table 3).
Comparison between CD44-positive and CD44-negative patients.
No: no p-value due to low number of cases in some subgroups.
Other parameters demonstrated non-significant differences. Nevertheless, four patients had positive MLL genes and all were CD44-positive. In addition, we observed that the MRD measured on D42 revealed ten suboptimal patients and all were CD44-positive. The last MRD revealed 11 suboptimal patients,who were all also CD44-positive. However, we could not compare these two groups due to the small number of cases in some subgroups (Table 3).
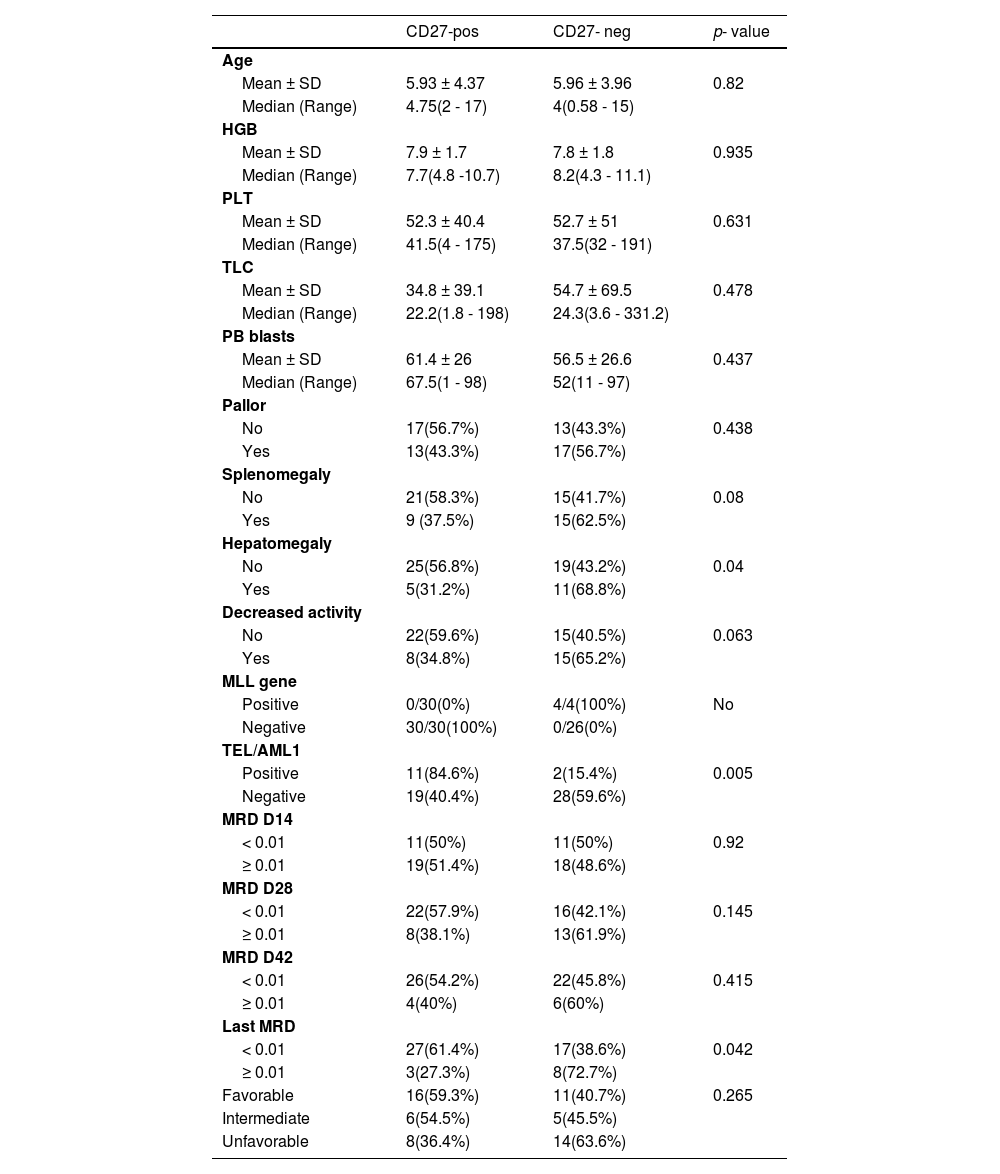
Comparisons between CD27-positive and CD27-negative patientsWe found a statistically significant difference between CD27-positive and CD27-negative patients with regard to hepatomegaly, as 16 cases of hepatomegaly presented with negative CD27 (68.8 %) (p = 0.04). There was a significant association between the CD27 and TEL/AML1 gene rearrangement, as 11 of 13 TEL/AML1-positive cases were CD27-positive (84.6 %; p = 0.005). Regarding the MLL gene and the CD27, all four MLL-positive patients were CD27-negative (Table 4).
Comparison between CD27-positive and CD27-negative patients.
No: no p-value due to low number of cases in some subgroups.
There was a significant difference between the CD27-positive and CD27-negative patients in terms of the MRD observed at the most recent visit, as 44 patients with MRD less than 0.01 were predominantly CD27-positive (61.4 %), whereas 16 patients with suboptimal MRD at 0.01, or over, were predominantly CD27-negative (72.7 %; p = 0.042). There were no significant differences regarding other parameters (Table 4).
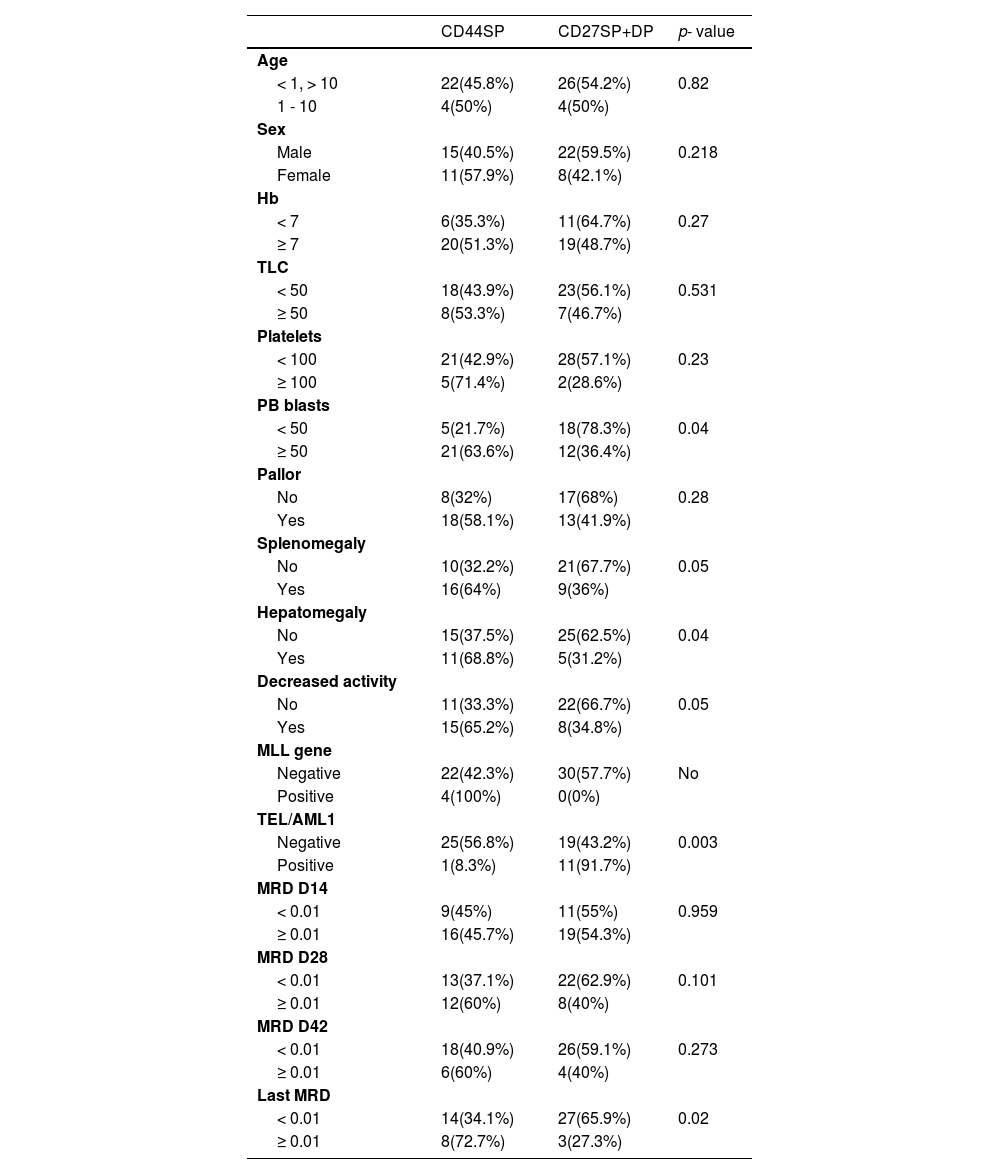
Comparisons between CD44 SP and group of CD27 SP and DP patientsThere was a significant difference regarding the PB blasts between the CD44SP and the CD27SP and DP group, as 78.3 % of patients with less than 50 blasts were in the CD27SP and DP group. Moreover, 63.6 % of the patients with 50, or more, blasts were CD44SP (p = 0.04). In addition, we found that most of the patients had hepatomegaly (68.8 %), splenomegaly (64 %) and decreased activity (65.2 %) and all were CD44sp (p = 0.04, 0.05 and 0.05, respectively (Table 5).
Comparison between CD44sp and the group (CD27SP, DP).
No: no p-value due to low number of cases in some subgroups.
In our study, we found a significant difference regarding the TEL/AML1, as only 8.3 % of the TEL/AML1-positive patients were CD44SP, and 91.7 % of them were in the CD27SP and DP group (p = 0.003). Regarding the MLL gene, we had four patients positive for it and all were CD44SP (Table 5).
A significant difference was reported between the CD44SP patients and those of the CD27SP and DP group regarding the MRD at their last visit, as 72.7 % of the patients with a suboptimum MRD at their last visit to the hospital were CD44SP. Only 65.9 % of the patients with the last MRD less than 0.01 were in the CD27SP and DP group (p = 0.02) (Table 5).
Minimal residual diseaseThe response to treatment was assessed by flow cytometry on days 14, 28 and 42 and at the last visit. The MRD with complete response (CR) (< 0.01) was detected in 22/59 patients (37.3 %) on day 14, in 38/59 patients (64.4 %) on day 28, in 48/58 patients (82.8 %) on day 42 and in 44/55 patients (80 %) at their last visit (Supplementary Table 3).
Mortality was reported in 5 patients, with two deaths occurring early (within the first 42 days of treatment). The first patient died during the induction phase of the treatment (day 15) due to brain death, the second died after one month of treatment due to septic shock and three died during follow-up. Two patients died at 18 months due to measles and pneumonia, respectively, and the last died after 24 months due to fungal infection (Supplementary Table 3). The cause of the first four deaths was determined to be CD44SP, while the final death was DP. Two patients receiving palliative care experienced a relapse. The first was DN and the second, CD44s.
Survival analysisOverall survivalThe average duration of the follow-up was 19.0 months (range, 0.33 - 27.7 months). The median overall survival was 26.5 months, the cumulative overall survival at one year was 96.6 % and at 24 months, 91.8 % (Supplementary Figure 1). There was no significant variation in the overall survival in our ALL patients regarding different expression patterns of our studied markers CD44 and CD27. However, there was a borderline higher overall survival for patients with DP and CD27SP, as we found the cumulative overall survival of the CD27SP and DP group at 26 months was 100 %, and that of CD44SP, 77.4 % (p = 0.08) (Supplementary Figure 2).
Event-free survivalRegarding the EFS, in addition to five mortalities, two patients relapsed, the first achieving CR after 110 days and then relapsing after five months. The second patient experienced complete remission after 14 days and relapsed after 100 days. Another cycle was taken, and until now, they are on palliative treatment. Consequently, the 1-year EFS was estimated to be 93.3 %. (Supplementary Figure 1). There was no significant variation in the disease-free survival in the ALL patients regarding different expression patterns of our studied markers CD44 and CD27.
DiscussionOver 90 % of the children diagnosed with ALL in the USA have been cured. Despite these high rates of recovery, some patients have a dismal prognosis. These patients continue to be challenging to treat, as their cure rates remain suboptimal.24 The adhesion molecule CD44 has been involved in lymphocyte activation, recirculation and homing, through extracellular matrix adhesion.25 The CD44 was highly expressed in pediatric T-ALL and AML.20,26 The CD44 was also associated with chemotherapy resistance in T-ALL, in which its expression was related to the efflux of drugs from the cell.27
The CD27 is a member of the TNF superfamily, which is expressed constitutively on activated effector T cells, NK cells and antibody-secreting plasma and memory B cells.28 The CD27 protein and mRNA expression have been reported in B-cell lymphomas.29 and adult T-cell leukemia/lymphoma.30 In acute myeloid leukemia, the CD27 is considered a prognostic biomarker.31
This study was hypothesized to study the clinical and prognostic significance of differential expression patterns of the CD27 and CD44 in 60 new Egyptian pediatric patients with B-ALL and validate the use of these markers in the MRD monitoring and their association with different stages of B-ALL development, maturational subtypes, genetic alterations, clinical characteristics and prognostic importance.
The majority of lymphoblasts in our ALL patients were positive for CD44, with a mean expression of 61.5 %. Nearly the same expression was observed in normal cells. The CD44 was broadly distributed in various cell types and tissues, which is consistent with the observations of prior research.25
Our study demonstrated a significant association between the CD44 positive expression and the presence of some unfavorable prognostic markers, such as decreased platelet count, higher TLC and higher PB blast percentage at presentation, as the CD44-positive patients have a significantly lower platelet count (26.6 ± 17.9) vs. (54.9 ± 46.7), p = 0.04, higher TLC (100 ± 129.9) vs. (39.6 ± 44.3), p = 0.01, and higher blast percentage (85 ± 11) vs. (56 ± 25), p = 0.011, than CD44-negative patients. No significant difference was detected regarding other well-established prognostic factors.
The same results were reported in previous studies by Amirghofran et al., who found significant differences between the TLC, PB blasts and CD44-positive and CD44-negative patients.32 Other reports found no significant correlation between the CD44 expression and standard risk factors, such as age, WBC count, central nervous system involvement, and chromosomal abnormalities.12
Our study reported no significant difference between the CD44 and TEL/AML1 gene rearrangement. In contrast, Amirghofran et al. found that sCD44 was higher in TEL/AML1 positive patients (p = 0.003).32 This difference can be attributed to the widespread presence of the CD44 on 91.6 % of blast cells. We reported a strong association between the MLL gene, regarding CD44-positive patients, as all four patients with the MLL gene were also CD44-positive. However, this association was not statistically significant due to the small number of patients.
There was no significant difference between the CD44-positive and CD44-negative patients in terms of MRD on D14, D28 and D42 and the most recent follow-up visits; however, on D42, we observed ten suboptimal CD44-positive patients and 11 suboptimal CD44-positive patients in the most recent MRD evaluation. There was no statistically significant difference between the cytogenetic groups CD44-positive and CD44-negative patients and previous study results.32
In our study, the CD27 was expressed in 50 % of the patients, with a median expression of 47.8 %, a highly significant difference from that of the control group (14.9 %). These findings are consistent with Mohseni et al., 2008, who found elevated CD27 expression levels in patients with ALL at diagnosis.33,34 Furthermore, Chen et al., 2017, found that the CD27 is highly expressed in both childhood and adult BCP-ALL.13
We reported a significant difference between the CD27-positive and CD27-negative patients regarding hepatomegaly, as we have observed 16 cases presenting with it, 11/16(68.7 %) of them being negative for CD27. These results align with Kamazani et al., 2012, who found that the mean CD27 expression was significantly higher in patients with less extramedullary involvement (p = 0.008).12 There were no significant differences regarding other well-established prognostic factors.
In addition, we found a significant association between the expression of the CD27 and TEL/AML1, 13 of our patients being TEL/AML1-positive and 84.6 % of them, positive for the CD27 expression (p-value = 0.005). These results agree with Vaskova and colleagues, who demonstrated an association between the CD27 and TEL/AML1-positive subgroup of childhood leukemia.34,35 The TEL/AML1-positive leukemia has a better outcome. Therefore, the association of the CD27 with this subtype suggests a vital role for the CD27 molecule in the host's anti-leukemic defense.24
We found a higher expression of the CD27 in low-risk patients and those who entered CR, 44 of our patients having an MRD less than 0.01. Most of them (61.4 %) are CD27-positive and, in the 16 patients with a suboptimal MRD, most of them (72.7 %) are CD27-negative (p-value 0.042). This suggests a role for the CD27 in anti-leukemic defense in ALL patients. It has been reported that the interaction of the CD27 with its ligand (CD70) on T-cells can activate these T-cells and stimulate immune responses against leukemic cells. In addition, it contributes to the induction of apoptosis in leukemic cell lines.12
Finally, the expression patterns of the CD27 and CD44 were investigated in relation to various prognostic factors. The CD44 positivity was associated with poor prognostic factors, such as high PB blasts over 50 %, hepatomegaly (68.8 %), splenomegaly (64 %) and decreased activity (65.2 %). This negative association between the CD44SP pattern, in contrast to CD27sp or DP pattern with remission induction observed in our study, may also suggest a role for the CD44 molecule in the biology of ALL. The CD44 molecule can act as a marker of metastasis. Nevertheless, it is also involved in lymphocyte activation (36). In contrast, the CD 27 positivity was significantly correlated with the favorable prognostic indices, such as lower PB blasts, less extramedullary involvement, negative MLL gene expression and positive TEL/AML1 expression, optimum MRD suggesting that the CD27 expression may be a potentially useful predictor of a good prognosis in patients with ALL.
ConclusionIn conclusion, our findings showed that the CD44 was expressed in the majority of the cells (91.6 %), while the CD27 was expressed in 50 % of the patients. The CD44 positivity was associated with some unfavorable prognostic factors, while the CD27 positivity was significantly correlated with favorable prognostic indices. When the CD44 positivity was accompanied by the CD27 positivity, the poor prognostic relevance of the CD44SP appeared to be counteracted. This finding may indicate a role for the CD27 in the host's anti-leukemic defense in ALL patients.
Different expression patterns of the CD27 and CD44 were associated with a number of known risk factors and therapeutic responses, indicating the biological significance of these molecules in ALL. These molecules may serve as a therapeutic target. Future studies involving a greater number of patients are recommended to examine the significance of these molecules in B-ALL.