Latin America is a very diverse region, but it has some common problems. To access diagnostic tests or high-cost drugs is difficult, because of slow and bureaucratic systems. Moreover, there are significant differences in access within countries (subsystems) with high out-of-pocket expenses.1
Chile has mainly two health systems, one public and one private. The private system has almost no restriction to drug or test accessibility. On the other hand, the public system has several problems to access diagnostic tools and newer drugs. Treatment of transplant-eligible patients with newly diagnosed multiple myeloma (TE-NDMM) has evolved; financed by the Ministry of Health since 2000; progress is divided into four treatment periods.
The main objective of this study was to evaluate the overall survival (OS) of TE-NDMM patients in the different periods.
This is an ambispective observational cohort study. A total of 213 under 65-year-old patients from our institutional registry were analyzed. Since 2013, the registry has been prospective. Four periods were defined:
- •
Period 1 (P1) from 2000 to 2007, in which only melphalan-prednisone was available for treatment;
- •
Period 2 (P2) from 2008 to 2013, in which thalidomide began to be used for some young patients in addition to autologous stem cell transplantation (ASCT) in some cases;
- •
Period 3 (P3) from 2014 to 2018, in which the protocol was cyclophosphamide-thalidomide-dexamethasone (CTD) and ASCT
- •
Period 4 (P4) from 2019 to 2022, in which triplets based on bortezomib, ASCT and post-transplant maintenance with lenalidomide were used.
Treatment in the different periods, early mortality (defined as death within the first six months of diagnosis), and OS of each group were analyzed.
In P1 there were 37 patients, 56 in P2, 61 in P3, and 59 in P4. In P1, 65 % of patients were treated with a melphalan-prednisone regimen, 8 % with VAD, and 8 % with a thalidomide-based regimen. No ASCT was performed and the early mortality rate was 24 %. In P2, 71 % of cases were treated with a thalidomide-based regimen (13 % CTD and 87 % thalidomide-dexamethasone), 20 % based on melphalan and 2 % based on bortezomib; 21 % performed ASCT and 9 % received thalidomide-based maintenance. Early mortality was 16 %. In P3, 90 % received a thalidomide-based regimen (82 % CTD) and 10 % based on bortezomib; 33 % underwent ASCT with 20 % receiving maintenance (100 % thalidomide-based); the early mortality rate was 16 %. In P4, 97 % of patients were treated with bortezomib-based triplets with 36 % being transplanted. Maintenance was administered to 75 % of patients, 93 % based on lenalidomide. Early mortality was 7 %.
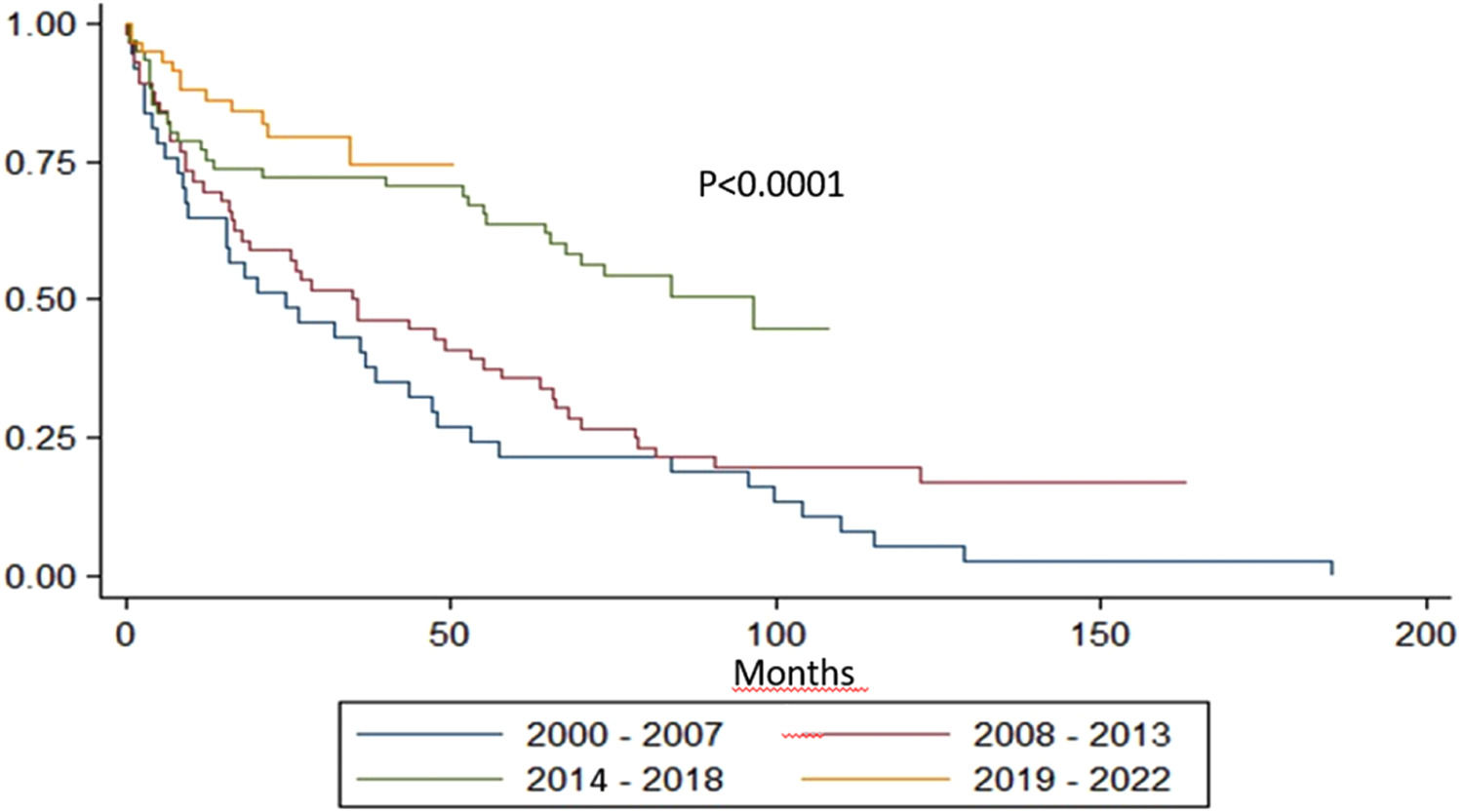
With a median follow-up of 77.5 months, the median OS for the entire group was 55.1 months. The median OS of P4, P3, P2 and P1 was not reached, 96, 35 and 25 months, respectively (p-value <0.0001 - Figure 1).
The OS increased in each treatment period. Induction regimens have gone from being melphalan-based, to thalidomide-based and then to being mostly bortezomib-based. Transplant and maintenance rates have increased over time and early mortality has decreased, especially in P4. The median OS in P3 was eight years. Of interest, the induction and maintenance regimens were mainly thalidomide-based in that period. P4 showed a trend of an even better OS that could have been investigated further with a longer follow up. These results were better than expected, and here we summarized what we think are the milestones that got us there (Table 1).
First, our center is a high-volume facility and an academic center, two variables that have been associated with better outcomes in cancer patients.2
In the public system, there are basically three forms of drug financing for TE-NDMM patients. The Explicit Health Guarantees program (GES in Spanish) applies to a group of diseases defined by the Ministry of Health that, by law, require timely and quality diagnosis and treatment. Multiple myeloma (MM) entered the GES pathology list in October 2019. Through GES we financed, among other things, bortezomib-based triplet regimens.
The second form is through the so-called “High-Cost Drugs Committee” of the Ministry of Health. Post-transplant maintenance lenalidomide is obtained since 2020 through this committee. Additionally, this committee gives bortezomib, lenalidomide and dexamethasone as second line to patients who have had non-bortezomib-based induction, and bortezomib-thalidomide-dexamethasone-cisplatin-doxorubicin-cyclophosphamide-etoposide (VTD-PACE) for aggressive cases or plasma cell leukemia.
The third way of financing drugs is local management by each center. Our center, with much effort and an understanding about how crucial it is to have rescue drugs, has incorporated daratumumab, carfilzomib and pomalidomide into its arsenal. Therefore, since 2021 we have been able to use these drugs in selected patients for refractory or relapsed MM.
Additionally, the national transplant program was created in 2010. Until 2019, there was only one public center that performed transplants. Recently another two transplant centers have opened in the public system (2019 and 2022), which could explain the increase in autologous transplantations for MM in the country.
On the other hand, in 2017 a MM patient association was created. This partnership has been critical for achieving goals, as previously reported.3 This association helped us in guiding treatment options and providing valuable information about patients’ priorities.
Also in 2017, a MM study group was created and hematologists from all over the country with special interest in monoclonal gammopathies met. In 2018 we published the largest retrospective observational study in Chile, with more than 1000 patients.4 Although it is not a prospective national registry, it helped us evaluate our shortcomings and weaknesses. It is known that population-based cancer registries have a critical role in epidemiologic surveillance of neoplasms, allowing policy makers to identify gaps.5
We also believe that MM awareness has increased in recent years, in part because of the continuing education that we provide at our center and with the MM study group, which has led to more patients being admitted to intensive care units, with access to newer and more powerful antibiotics and to hemodialysis.
In conclusion, different policies in the Chilean public system have improved the OS in TE-NDMM patients at our center. It can be an example for other countries to take action.