Primary myelofibrosis is a Philadelphia-negative myeloproliferative neoplasm characterized by clonal myeloid expansion, followed by progressive fibrous connective tissue deposition in the bone marrow, resulting in bone marrow failure. Clonal evolution can also occur, with an increased risk of transformation to acute myeloid leukemia. In addition, disabling constitutional symptoms secondary to the high circulating levels of proinflammatory cytokines and hepatosplenomegaly frequently impair quality of life. Herein the main current treatment options for primary myelofibrosis patients are discussed, contemplating disease-modifying therapeutics in addition to palliative measures, in an individualized patient-based approach.
Primary myelofibrosis (PMF) is a Philadelphia-negative myeloproliferative neoplasm (MPN) with a predominant proliferation of megakaryocytes and granulocytes in the bone marrow characterized by an initial proliferative phase, followed by a reactive deposition of fibrous connective tissue in the terminal phase.1 Bone marrow failure, thromboembolic events and transformation to acute myeloid leukemia (AML) are the main causes of morbi-mortality in PMF, but additional symptoms secondary to hepatosplenomegaly and abnormal blood counts frequently impair quality of life.1,2 The high circulating levels of proinflammatory cytokines also result in disabling constitutional symptoms (fatigue, weight loss, night sweats, fever, pruritus, arthralgias, myalgias).2 Hence, the decision regarding the best treatment combination in PMF must be individualized, taking the symptoms, risks and life expectation of each patient into account. Despite the recent advances in the development of targeted therapies, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the only curative option available for PMF. Evidence on the main therapeutic options for PMF will be discussed in this article.
Molecular characterizationAlthough no molecular lesion can specifically identify PMF, some recurrent mutations are found and are helpful in the diagnosis and the prognostic stratification of PMF patients. JAK2 (Janus kinase 2), MPL (thrombopoietin receptor) and CALR (calreticulin) genes frequently harbor somatic mutations in PMF, which induce the constitutive activation of the JAK-STAT, PI3K and ERK pathways in a ligand-independent way, leading to increased myeloid proliferation. Approximately 50–60% of PMF patients exhibit the JAK2V617F mutation.3–5 A gain-of-function mutation in MPL (MPLW515K/L), which encodes the thrombopoietin receptor and is a key factor for growth and survival of megakaryocytes, has been reported in up to 5% of PMF cases.6,7MPL mutations may occur concurrently with the JAK2V617F mutation.8 Approximately 60–80% of JAK2 and MPL wild type patients harbor CALR mutations.9,10 Additional mutations in epigenetic regulators, such as TET2,11ASXL1,12DNMT3A,13IDH1/2,14 have been described in MPN patients at variable frequencies and their prognostic value has been object of studies.15
Risk stratificationAdequate risk stratification in PMF is essential to establish the most suitable treatment for a particular patient, taking the risk-benefit of each approach into account. In this sense, the Dynamic International Prognostic Scoring System (DIPSS) for PMF is widely used in the clinical practice. DIPSS is a dynamic prognostic model that considers modifications in the risk profile after diagnosis and can predict prognosis at different stages of the disease (Table 1).16 The age-adjusted DIPSS is a variation specifically developed for younger patients (age <65 years), comprising the group that is most commonly suitable for intensive therapies such as allo-HSCT (Table 1).16
Risk stratification of primary myelofibrosis patients according to the Dynamic International Prognostic Scoring System (DIPSS) and the age-adjusted DIPSS (aaDIPSS)16
| DIPSS | aaDIPPS | |||||
|---|---|---|---|---|---|---|
| Value | Value | |||||
| 0 | 1 | 2 | 0 | 1 | 2 | |
| Age (years) | ≤65 | >65 | – | – | – | |
| White blood cell count (×109/L) | ≤25 | >25 | ≤25 | >25 | ||
| Hemoglobin (g/dL) | ≥10 | <10 | ≥10 | <10 | ||
| Peripheral blood blasts (%) | <1 | ≥1 | <1 | ≥1 | ||
| Constitutional symptomsa | No | Yes | No | Yes | ||
Hydroxyurea (HU) is a non-alkylating antineoplastic agent used for cytoreduction in myeloproliferative neoplasms. Although there are few well designed studies evaluating HU benefits in myelofibrosis patients, hydroxyurea is frequently used to attenuate hyperproliferative manifestations related to PMF.17 In a group of 40 PMF patients, Martinez-Trillos et al. showed significant response rates, with reductions in constitutional symptoms (55%), symptomatic splenomegaly (45%), thrombocytosis (40%) and leukocytosis (28%); accentuation of anemia was the most common adverse event, and was observed in almost half of the patients.17 When HU resistance/refractoriness is documented in the PMF proliferative phase, switching from HU to a molecular targeted therapy (i.e., JAK1/2 inhibitor) should promptly be considered.18 The criteria for resistance and refractoriness to HU in PMF patients have previously been defined by the European LeukemiaNet consensus.18
Support therapyAnemiaAnemia is a frequent manifestation of PMF19 that might be caused by different interacting factors, such as bone marrow insufficiency (fibrosis), hypersplenism, bleeding, iron deficiency, vitamin B12 or folate deficiency, or autoimmune hemolysis.20,21 Moreover, specific PMF treatment with cytoreductive drugs (HU)17 and JAK1/2 inhibitors22 can lead to, or increase, anemia in these patients. Besides correcting the potentially reversible causes of anemia, some other therapeutic possibilities might be considered when anemia is a disabling symptom. Some of them are discussed below.
a. Androgens
Androgens have been used to treat anemia in PMF with variable response rates; most of the studies described results observed in small cohorts. Danazol, a semisynthetic attenuated androgen that has fewer side effects, results in an anemia response rate of 30–57% depending on the adopted response criteria.21,23,24 In a cohort of 50 patients with PMF, Cervantes et al.21 described a 30% response rate [defined by transfusion cessation in transfusion-dependent patients or an increase in hemoglobin (Hb) >2g/dL in patients without transfusion requirements], with a median duration of anemia response of 14 months. Androgens should not be used in patients with prostatic symptoms, prostate cancer or moderate to advanced hepatic disease.
b. Erythropoiesis-stimulating agents
Although recombinant human erythropoietin (EPO) has been widely used for the treatment of anemia of a variety of causes, the experience in PMF is relatively small and based on studies with limited sample sizes. Reported overall response rates range from 23% to 60%.25–28 Hb <10g/dL, transfusion independence,25,27 and EPO levels <125U/L27 are factors that might confer a better response to treatment. However, these data need to be validated in larger cohorts using uniform response criteria.
c. Immunomodulating drugs
Thalidomide has shown effectiveness in a wide spectrum of neoplasms due to its anti-angiogenic and immunological effects. Previous reports using high doses of thalidomide (100–400mg) in PMF have demonstrated encouraging responses regarding the improvement of anemia (20%–60%), thrombocytopenia (38%–80%), and splenomegaly (25%–41%).29–32 However, the high level of side effects (somnolence, fatigue, edema, constipation, neurological symptoms, neutropenia) significantly reduced tolerability.29,30,32,33 The use of low doses of thalidomide (50–100mg) associated or not with prednisone (0.5mg/kg/day) can decrease toxicity with similar response rates.34–36 Lenalidomide, a thalidomide analog, has been described as an additional therapeutic option, with response rates ranging from 19–30% for anemia, 0–50% for thrombocytopenia and 10–42% for splenomegaly,37–39 according to the scheduled dose and the response criteria adopted. Recently, Daver et al.40 evaluated the combined effect of lenalidomide plus the JAK1/2 inhibitor ruxolitinib in 31 patients with PMF; however, the study had to be discontinued prematurely due to the elevated number of drop outs (23 patients) due to drug toxicity.40 Among the patients who did not require early dose interruption, response rates were high (73%), suggesting that additional studies using lower doses of lenalidomide and ruxolitinib might be of interest.40 Moreover, a retrospective study by Jabbour et al.41 that evaluated three previous phase 2 clinical trials compared the efficacy of thalidomide and lenalidomide-based therapies, and observed overall response rates of 16% and 34–38%, respectively, according to the International Working Group (IWG) for Myelofibrosis Treatment and Research criteria. In addition, lenalidomide plus prednisone improved response duration when compared to both thalidomide and lenalidomide single agent therapies, suggesting that lenalidomide plus prednisone might be a reasonable option when deciding for immunomodulating drugs.41 Pomalidomide is a potent second-generation immunomodulating drug that has a better toxicity and safety profile than thalidomide and lenalidomide,42 and has been tested in PMF in few studies with varying results. Anemia response rates ranged from 10–37%,42–47 but increased to 53% in a single report that specifically analyzed the group of JAK2V617F-positive patients with <10cm palpable splenomegaly and <5% circulating blasts, indicating that these factors might be predictors of better response.44
d. Chronic transfusion and iron chelation
For the symptomatic anemic patients that are refractory to specific therapies, chronic red blood cell transfusions may improve symptoms and quality of life. For the treatment of secondary iron overload in transfusion-dependent patients who have curative intentions (e.g. bone marrow transplantation) and/or higher life expectations, iron chelation should be considered in order to prevent iron-induced organ damage. Furthermore, iron chelation may promote an erythroid response with increased Hb levels in some PMF patients,48 albeit additional studies are needed to support this recommendation.
SplenomegalyThe enlarged spleen is a major source of discomfort and impaired quality of life in PMF. Constitutional symptoms, pain, early satiety due to gastric compression, portal hypertension and cytopenias are frequent findings in PMF in the fibrotic phase.49,50 In PMF patients with an unsatisfactory response to pharmacological treatment, splenectomy and splenic radiation may be treatment options, as discussed below.
a. Splenectomy
Despite the improvement in perioperative mortality following splenectomy observed in the last decades due to better patient selection, vaccination, antimicrobials and surgical procedures, splenectomy does not seem to alter patient survival and disease evolution.49 However, it might be exceptionally indicated for the palliative control of persistent anemia, thrombocytopenia, portal hypertension and pain.49,51 In a retrospective analysis of 223 patients submitted to splenectomy at the Mayo Clinic during a 20-year period, durable remissions in constitutional symptoms, transfusion-dependent anemia, portal hypertension, and severe thrombocytopenia were achieved in 67%, 23%, 50%, and 0% of the patients, respectively, with rates of nonfatal complications of 30.5%, including 7.2% of thrombosis, and 8.9% of fatal complications.51 Santos et al.52 described, in a cohort of 94 splenectomized PMF patients, improvements of anemia and thrombocytopenia in 47% and 66% of the cases, respectively. Thrombosis was observed in 16% of the patients, and post-operative mortality was 5%, with a lower overall survival for the patients that were submitted to splenectomy during disease evolution.52 Since splenectomy is associated with substantial risks, the procedure should only be considered for selected patients following stringent criteria including absence of severe comorbidities, adequate life expectancy, significant splenic symptoms that affect quality of life, and failure of at least one pharmacological therapy for splenomegaly.49
b. Splenic irradiation
Splenic irradiation is a palliative modality of treatment considered as an alternative to splenectomy in PMF patients that have symptomatic splenomegaly and are ineligible for surgical procedures. However, although spleen size reduction and symptom relief are observed in a high proportion of patients, response to treatment is usually brief and transient.50,53 The worsening of pre-treatment cytopenias and the emergence of infectious complications are frequently found.53
Additional palliative measuresMyelofibrosis severely compromises quality of life as a result of marked splenomegaly, profound constitutional symptoms and cachexia.54 In a group of 458 patients, including PMF and post-polycythemia vera (PV)/post-essential thrombocytopenia (ET) myelofibrosis, Mesa et al. found an incidence of 84% for fatigue, 47% for bone pain, 50% for pruritus, 56% for night sweats and 54% for symptomatic splenomegaly.54 Therefore, adequate symptom control, such as optimized pain management and evaluation of nutritional status, are mandatory adjuvant therapies.
InterferonInterferon has been described as a therapeutic option in the treatment of PMF patients, though only a limited number of studies evaluating the effects of interferon in larger PMF cohorts are available. Previous studies indicated that recombinant interferon-α has the potential to decrease the proliferation of PMF neoplastic stem cells with a significant reduction of marrow fibrosis, cellularity and megakaryocyte density,55,56 which can result in improvements of splenomegaly and blood counts.56,57 Systemic toxicity (cytopenias, asthenia, fatigue, myalgia) may limit interferon use in a proportion of patients; however, most cases can be manageable with dose reductions.55,56 Larger studies are necessary to fully support interferon use in PMF patients and its effects on overall survival.
JAK1/2 inhibitorsThe activation of the JAK-STAT pathway induced by mutations in JAK2, CALR and MPL genes has a central role in inducing cell proliferation in PMF,15 making this pathway a potential target for directed therapies. Ruxolitinib, the first US Food and Drug Administration (FDA)-approved oral JAK1/2 inhibitor is generally well-tolerated, and significantly reduces splenomegaly and ameliorates debilitating myelofibrosis-related symptoms.22,58 An evaluation of the nutritional status in intermediate-2 or high risk PMF patients showed that ruxolitinib significantly increased weight and albumin levels.59 The most common side effects are a dose-dependent anemia and thrombocytopenia, that are usually manageable with dose reductions.22,58 A three-year follow up analysis of the controlled myelofibrosis study with oral JAK inhibitor (COMFORT-I) – a double-blind, placebo-controlled trial that previously suggested a survival benefit for the ruxolitinib group58 – reported that ruxolitinib significantly improved quality of life, reduced spleen volume and improved survival of patients with intermediate-2 or high risk PMF when compared to placebo.60 However, a recent Cochrane meta-analysis suggested that there is insufficient data for definitive conclusions regarding the benefits of ruxolitinib on the survival of PMF patients.61 Future updates in ruxolitinib studies showing longer follow-up times will be of value to allow definitive conclusions regarding survival benefits. In order to identify genes that may predict response to ruxolitinib in myelofibrosis patients, Patel et al.62 screened mutations in 28 genes recurrently mutated in hematologic malignancies, and found that patients with ≥3 mutations had poorer responses to ruxolitinib and shorter overall survival.62 Despite other selective JAK inhibitors having been submitted to clinical trials with promising results63,64 none have been approved for clinical use until the present.
Bone marrow transplantationAllo-HSCT is currently the only curative treatment option for myelofibrosis patients. Allo-HSCT should be considered in intermediate-2/high risk patients, and in patients with refractory disease, adverse cytogenetics or >2% blasts in the peripheral blood.65–68 Although age >45 years is described as an adverse prognostic factor for transplantation in PMF,66 allo-HSCT can be considered for individuals younger than 70 years old who have good performance status and no significant comorbidities.65 Previous studies have demonstrated that unrelated donor, post-transplant transfusion dependence,66 and JAK2V617F levels >1% one month after transplantation67 are prognostic factors that adversely affect overall survival.
Special situationsPost-polycythemia vera and post-essential thrombocythemia myelofibrosisPV and ET are MPN which can evolve to myelofibrosis as a disease-related complication,69 with an incidence of evolution to fibrosis over 15 years of 5–14%69,70 and 9.3%,69,71,72 respectively. Post-PV and post-ET myelofibrosis are molecularly distinct but phenotypically similar to PMF, with equivalent clinical courses; for this reason, most clinical trials group patients of these three disease categories in their cohorts.2 Currently, there are no specific therapeutics for post-PV and post-ET myelofibrosis, and these patients should be treated similarly to PMF patients.2,73
Autoimmune myelofibrosisAutoimmune myelofibrosis (AMF) is a benign cause of bone marrow fibrosis associated with autoimmune disorders, such as systemic lupus erythematosus, scleroderma, Sjogren's syndrome, Hashimoto thyroiditis, autoimmune hepatitis, and Evans syndrome.74 AMF can also be found in patients with no well-established diagnosis of autoimmune disease, usually associated with elevated titers of antinuclear antibodies, rheumatoid factor, and/or a positive direct antiglobulin test.74,75 It is characterized by reticulin marrow fibrosis in the absence of clustered or atypical megakaryocytes or other clinicopathological features of hematological malignancies.76 AMF usually responds to corticosteroid therapy with a generally good prognosis.74,76 A course of prednisone starting at 1mg/kg/day and tapered over 1–3 months can result in complete normalization of peripheral blood counts.76,77 Cases with partial response to corticosteroids appear to benefit from the addition of another immunosuppressive agent. In general, results of steroid therapy have been less impressive in AMF associated with systemic lupus erythematosus. Although reduction of bone marrow fibrosis after immunosuppressive treatment may be observed, the complete resolution of bone marrow fibrosis is not necessary for the recovery of peripheral blood cytopenias.76 Considering the differences in treatment and prognosis, the differential diagnosis between primary myelofibrosis and autoimmune myelofibrosis is crucial.
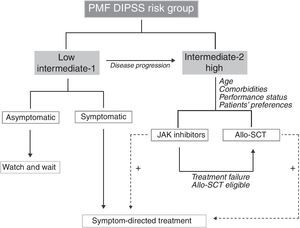
Treatment decision – when and whom?Asymptomatic PMF patients in the low and intermediate-1 risk groups according to DIPSS have a long expected survival and do not usually require specific treatment. If symptomatic, patients in these risk categories can be treated according to the prevailing symptom, as discussed above. Intermediate-2 and high-risk DIPSS patients have a shortened survival and should be considered for disease-modifying therapies, such as JAK inhibitors and allo-HSCT, when tolerated; the decision between these two options has to be individualized taking into account the patient's age, performance status and preferences. Combination therapies for the palliation of symptoms, regardless of the risk group and the prognosis, are beneficial and should be implemented to improve patients’ quality of life. A treatment algorithm is proposed in Figure 1.
Proposed treatment algorithm for primary myelofibrosis patients according to DIPSS risk groups. Therapeutic decisions take the risk group and patients’ particularities into account. The palliation of symptoms needs to be continuously pursued, independently of the therapeutic choice, and are additive to disease-modifying treatment, as indicated by the dotted lines. PMF: primary myelofibrosis; DIPSS: Dynamic International Prognostic Scoring System; Allo-HSCT: allogeneic stem cell transplantation.
Expressive progression has recently been achieved in the knowledge of the pathophysiology of primary myelofibrosis, which has allowed the development of targeted-drugs that may alter disease progression. Many molecule-specific drugs are under development or being tested, but could not be discussed herein due to space limitations. JAK inhibitors have shown promising results, though additional studies and follow-up time will be of value to further support survival benefits. Currently, allo-HSCT remains the only curative option. In a patient-based approach, the palliation of the symptoms is fundamental from diagnosis until end-stage disease.
Conflicts of interestThe author declares no conflicts of interest.
The author would like to thank Raquel S. Foglio for English review, and Dr. Sara T. Olalla Saad from the University of Campinas and Dr. Fabiola Traina from the University of São Paulo at Ribeirão Preto Medical School for their valuable collaboration.