Essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF) are Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) characterized by increased myeloid proliferation. The gain of function induced by the Janus kinase 2 mutation, JAK2V617F, has been reported in most PV and in more than half of ET and PMF cases.1 However, the presence of different disease phenotypes and the absence of the JAK2 mutation in some MPNs suggests that additional genetic lesions or/and aberrant signaling pathways may be involved in the pathogenesis of these diseases.1,2
In December 2013, somatic mutations in the calreticulin (CALR) gene were identified in ET and PMF patients by two independent groups3,4 and confirmed by others.5–9CALR mutations have been reported as mutually exclusive with JAK2 and MPL mutations and may be present in 56–88% of JAK2/MPL-negative cases.3,4 A recent paper reported a patient that had both mutations, JAK2V617F and a CALR exon 9 mutation, simultaneously.10 Over thirty different CALR mutations in exon 9 have been described, but the most frequent mutations (about 80%) may be classified as type-1 [L367fs*46; deletion of 52 base pairs (bp)] and type-2 (K385fs*47; insertion of 5bp).3,4 The functional changes induced by the mutations are still not completely elucidated, but the overexpression of the type 1 CALR mutation in Ba/F3 cells (an IL3 dependent cell line) leads to cytokine-independent cell growth and STAT5 activation.4 Gene expression signature studies also indicate that JAK2 and CALR mutations share mechanisms of malignant transformation, reaffirming a central role of the JAK/STAT signaling pathway in the pathogenesis of MPN.11
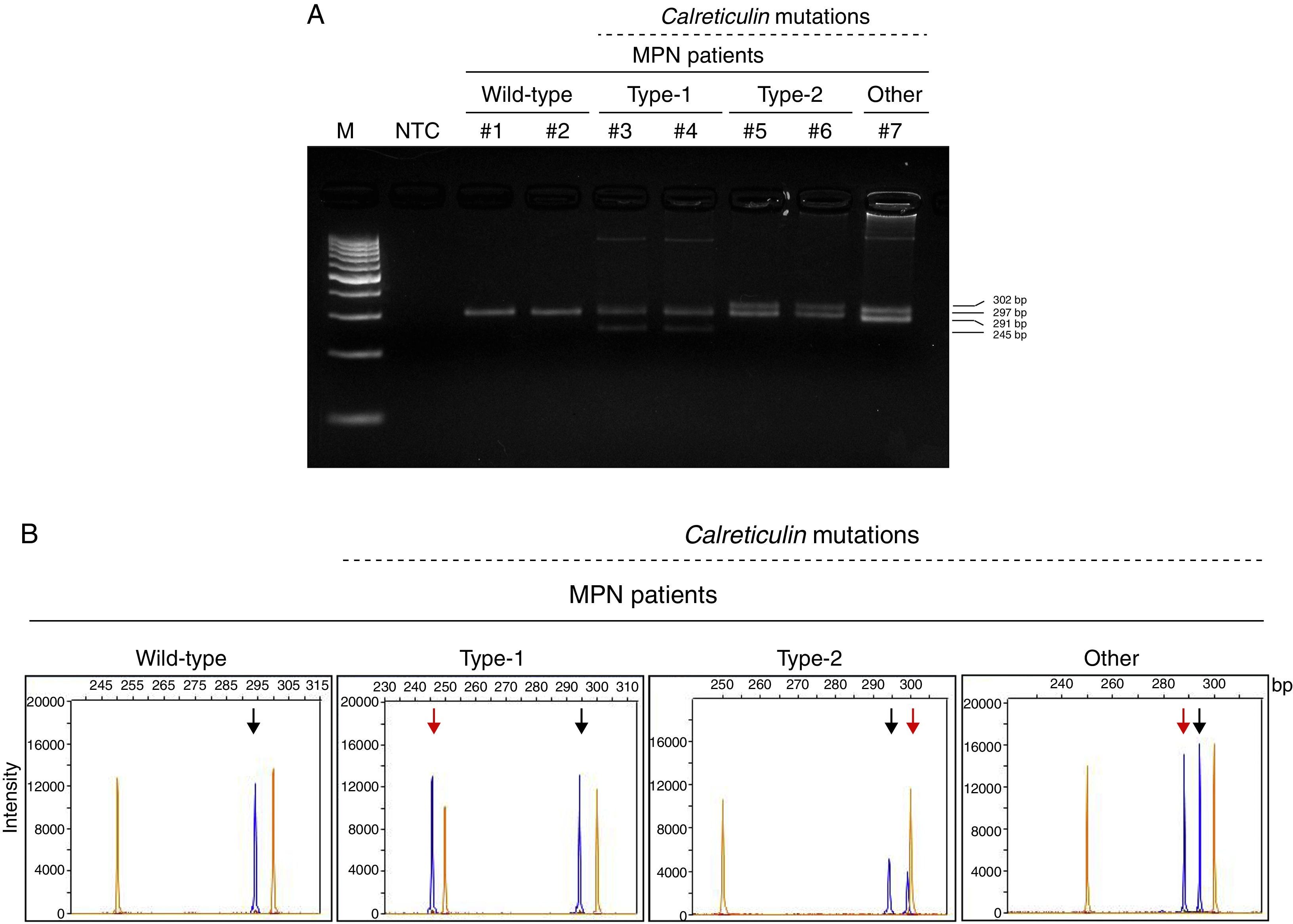
The aim of the present study was to characterize the prevalence of CALR mutations and the clinical and laboratorial characteristics of CALR-mutated patients in a Brazilian cohort of MPNs. Seventy-three MPN patients were included in the study (ET=32, PV=20, PMF=21). Patients’ characteristics are described in Table 1. Peripheral blood samples were collected, submitted to hemolysis, and DNA was extracted by the phenol/chloroform method. All samples were investigated for JAK2 and CALR mutations. The JAK2V617F mutation and CALR exon 9 mutations were verified as previously described.12,13CALR mutations were classified as type-1 (deletion of 52bp), type-2 (insertion of 5bp) or others (Figure 1).
Patient characteristics.
| MPN (n=73) | ET (n=32) | PV (n=20) | PMF (n=21) | |
|---|---|---|---|---|
| Age (years) – median (range): | 57 (19–87) | 53 (19–83) | 70 (41–88) | 62 (27–87) |
| Gender – male/female | 30/43 | 10/22 | 9/11 | 11/10 |
| Hemoglobin (g/dL) – median (range) | 13.9 (8.1–22.7) | 13.6 (9.7–17) | 18.4 (12.8–22.7) | 12.5 (8.1–19.9) |
| WBC count (×109L–1) – median (range) | 9.8 (2.4–43.2) | 9 (3.0–17.8) | 11.2 (6.3–25.5) | 11.8 (2.4–43.2) |
| Platelet count (×109L–1) – median (range) | 764 (108–2065) | 914 (541–2065) | 609 (203–1070) | 661 (108–1716) |
| JAK2 mutation status – n (%) | ||||
| JAK2WT | 28 (38) | 20 (63) | 0 (0) | 8 (38) |
| JAK2V617F | 45 (62) | 12 (37) | 20 (100) | 13 (62) |
| CALR mutation status – n (%) | ||||
| CALRWT | 53 (73) | 19 (59) | 20 (100) | 14 (67) |
| CALRMUT | 20 (27) | 13 (41) | 0 (0) | 7 (33) |
MPN: myeloproliferative neoplasms; ET: essential thrombocythemia; PV: polycythemia vera; PMF: primary myelofibrosis; WBC: white blood cell count; JAK2: Janus kinase 2 gene; WT: wild-type; CALR: Calreticulin gene; MUT: exon 9 mutations.
Calreticulin (CALR) indel mutations in myeloproliferative neoplasm (MPN) patients. (A) PCR amplification of exon 9 of CALR gene loaded on 4% agarose gel; lane 1: 100bp marker (M); lane 2: no template control (NTC); MPN patients #1 and #2: CALRWT; #3 and #4: heterozygotes for type-1 CALR mutation (CALRWT amplicons: 297bp; type-1 CALRMUT amplicons: 245bp); #5 and #6: heterozygotes for type-2 CALR mutation (CALRWT amplicons: 297; type-2 CALRMUT amplicons: 302bp); #7: heterozygote for non-type-1 or -2 CALR mutation (CALRWT amplicons: 297bp; CALRMUT amplicons: 291bp). (B) Representative PCR fragment size analysis of CALR amplicons from MPN patients: the orange peaks represent the GeneScan 500 LIZ dye Size Standard and blue peaks represent the CALR amplicons; the black arrows indicate the wild-type allele (297bp) and the red arrows indicate the indel-mutation alleles.
In our cohort, CALR mutations were found in 20 patients (13 ET and 7 PMF; Table 1) and were mutually exclusive with JAK2V617F: 20/73 (27%) of total MPN patients and 20/28 (71%) of the JAK2WT patients. Among the CALR-mutated patients, Type-1 CALR mutations were found in 50% (10/20; 8 ET and 2 PMF), type-2 in 40% (8/20; 4 ET and 4 PMF), and others in 10% (1 ET and 1 PMF) of the individuals. CALR mutations were not detected in PV patients (all JAK2V617F positive). In ET, CALRMUT patients showed reduced hemoglobin levels compared with JAK2V617F patients (p-value <0.01; Table 2); no differences were observed in white blood cell, neutrophil and platelet counts, thrombotic events, hepatomegaly, splenomegaly and constitutional symptoms. In PMF patients, CALR mutational status was not associated with clinical features (Table 2). The frequency of CALR mutations in this Brazilian cohort was similar to previously described frequencies.3,4,7 Furthermore, CALR and JAK2V617F mutations were mutually exclusive. Knowledge regarding the clinical impact of CALR mutations in MPNs is still under construction, but some studies indicate that CALR-mutated patients have lower ages at disease onset, lower hemoglobin and platelet counts, and have better overall survival than either JAK2-mutated or CALR/JAK2/MPL wild-type patients.5,9,14 In our cohort, CALRMUT ET patients presented lower hemoglobin levels, compared with JAK2V617F ET patients, even though in both groups hemoglobin values remained within the reference range.
Clinical and laboratory features of essential thrombocythemia and primary myelofibrosis patients, stratified according to JAK2 and CALR mutational status.
| Essential thrombocythemia | Primary myelofibrosisb | |||||||
|---|---|---|---|---|---|---|---|---|
| CALRMUT | JAK2V617F | CALRWT/JAK2WT | p-valuea,c | p-valuea,d | CALRMUT | JAK2V617F | p-valuea,c | |
| Gender: male/female – n (%) | 4 (31)/9 (69) | 5 (42)/7 (58) | 1 (14)/6 (86) | 0.69 | 0.61 | 4 (57)/3 (43) | 6 (46)/7 (54) | 1.00 |
| Age – years (range) | 57 (20–82) | 55 (44–83) | 43 (19–53) | 0.61 | 0.13 | 53 (27–80) | 65 (43–87) | 0.30 |
| Hemoglobin – g/L (range) | 13 (10–15) | 14 (12–17) | 14 (12–16) | 0.007 | 0.12 | 12 (9–13) | 14 (8–20) | 0.08 |
| White blood cells – ×109L–1 (range) | 7.1 (3–17.8) | 9.2 (4.5–16.7) | 9 (5–11.8) | 0.64 | 0.69 | 9.4 (2.4–35.7) | 11.8 (8.4–43.2) | 0.38 |
| Neutrophils – ×109/L (range) | 4.8 (2.1–15.1) | 6 (2.5–12) | 5.6 (3.2–8.5) | 0.43 | 0.48 | 6.2 (1–22.5) | 7.1 (0.5–38.3) | 0.48 |
| Platelets – ×109/L (range) | 938 (593–2065) | 880 (541–1340) | 984 (617–1274) | 0.37 | 0.87 | 567 (108–1716) | 661 (129–1606) | 0.69 |
| Thrombotic events – n (%) | 0 (0) | 2 (17) | 0 (0) | 0.50 | 1.00 | 0 (0) | 1 (8) | 1.00 |
| Hepatomegaly – n (%) | 1 (8) | 0 (0) | 0 (0) | 1.00 | 1.00 | 0 (0) | 2 (17) | 1.00 |
| Splenomegaly – n (%) | 2 (15) | 1 (8) | 1 (14) | 1.00 | 1.00 | 5 (71) | 7 (54) | 0.64 |
| Constitutional symptoms – n (%) | 1 (8) | 3 (25) | 2 (29) | 0.32 | 0.27 | 1 (14) | 3 (23) | 1.00 |
In conclusion, CALR mutations are highly frequent in Brazilian patients with MPN. The search for CALR mutations may be a useful tool for MPN diagnosis and further research on this mutation in Brazilian patients would be important to define the local incidence of the mutation, to improve diagnosis and classification of our patients, and to better evaluate the impact of these mutations on the outcomes of MPN patients.
Conflicts of interestThe authors declare no conflicts of interest.