The ABO blood group system plays an important role in transfusion and transplantation practices. Correct identification of the blood group of an individual is of paramount importance as minor errors can lead to fatal transfusion reactions. Weak expression of A, B and H antigens on the red cell surface can be inherited or acquired. They are probably the least frequently encountered and pose a challenge in the routine immunohematology practice.1 Such antigenic changes in hematological malignancies most commonly involve the ABO blood group system.2 The strength of agglutination with anti-B, anti-A,B, and anti-H serum, presence or absence of ABO isoagglutinins in the serum, adsorption-elution studies and the presence or absence of A, B and H substance in saliva along with pedigree analysis help to characterize the weaker variants of A and B serologically. Possible phenotypic subgroups include A3, Aend, Ax, Am, Ay, and Ael. Subgroups of B are very rare, less frequent than those of A and are classified as B3, BX, Bm and Bel in decreasing order of the amount of the B antigen (Table 1).
Serological characteristics of B phenotypes.1
| Phenotypes | Anti-A | Anti-B | Anti-A,B | Anti-H | Antibodies in serum | Substances in saliva |
|---|---|---|---|---|---|---|
| B | 0 | 4+ | 4+ | 2+ | Anti A | B, H |
| B3 | 0 | 2+mf | 2+mf | 3+ | Anti A | B, H |
| Bx | 0 | Wk | 3+ | Anti A, weak anti B | H | |
| Bm | 0 | 0/wk | 0/wk | 3+ | Anti A | B, H |
| Bel | 0 | 0 | 0 | 3+ | Anti A, sometimes weak anti B | H |
0: no agglutination; mf: mixed field agglutination; wk: weak agglutination.
Here we would like to share a case of the B3 blood group in a patient who was recently diagnosed with acute myeloid leukemia (AML) M3 with no known comorbid illness. To the best of our knowledge, this is the first report from India.
Case reportA 50-year-old gentleman was admitted to our tertiary care center with a one-month history of generalized weakness. A complete blood count revealed Hb: 7.9g/dL, platelet count: 20,000/μL and total WBC count: 148,300/μL. Peripheral smear examination revealed 90% atypical cells suggestive of acute leukemia and flow cytometric immunophenotyping of the peripheral blood sample confirmed AML-M3.
Blood grouping was done using the standard tube technique and column agglutination technology (Ortho Diagnostics, USA). Forward/cell grouping was performed using two different batches of monoclonal anti-A, anti-B, anti-A,B (Eryclone, Tulip diagnostics, Goa, India), anti-D antisera (Rhofinal, Tulip diagnostics, Goa, India) and anti-H lectin (Erybank, Tulip diagnostics, Goa, India) reagents and reverse/serum grouping was done using in-house prepared pooled group A1, B and O reagent red cells. The blood group was interpreted based on the agglutination in forward and reverse grouping (Table 2).
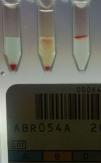
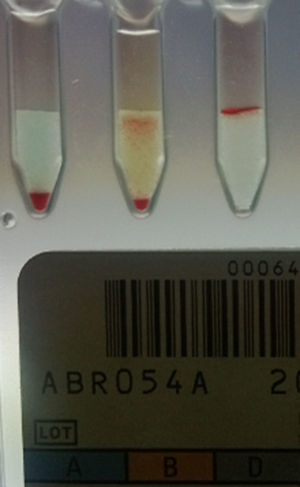
Forward grouping using the tube technique showed mixed field appearance with anti-B and anti-A,B antisera. Incubation of the forward grouping tubes at 4°C showed 3+/mf agglutination reaction with anti-B and anti-A,B antisera while reverse grouping results remained the same. Repeat blood grouping was carried out by the tube technique with another fresh sample and it was found to be no different to the previous results. Results were confirmed by column agglutination technology (ORTHO BioVue™, Ortho Clinical Diagnostics, USA) (Figure 1). His blood group was B Rh D positive as per his previous records.
The adsorption and elution test was performed as described in the American Association of Blood Banks (AABB) technical manual.3 Adsorption of patient red cells was done at 4°C with human polyclonal anti-B from group A individuals. Heat elution was performed at 56°C for 10min. Eluate and final wash supernatant were tested with three unpooled A, B and O blood group reagent red cell samples. Eluate reacted only with group B cells whereas final wash supernatant was non-reactive with A, B or O cells.
His saliva secretor status was determined using the hemagglutination inhibition test as described in the AABB technical manual.4 B and H antigens were absent in his saliva and so he was a non-secretor. According to the standard terminology of ABO subgroups, his blood grouping and Rh typing was B3 Rh D positive. Since he had a low platelet count, single donor apheresis platelets of group B were transfused; the procedure was uneventful. ABO discrepancy in his blood grouping was weak B antigen expression with mixed field appearance. This is an example of an acquired variant of B (secondary to the underlying myeloid leukemia).
DiscussionThe ABO gene is located on chromosome 9 and consists of seven exons with the majority of the coding sequences in exon 6 and 7. The amino acid substitutions within exon 7 determine whether glycosyltransferase will use UDP-N-acetyl-d-galactosamine (A transferase) or UDP-d-galactose (B transferase) donor sugars to synthesize A or B antigens, respectively. The nucleotide deletion in exon 6 that results in the inactive enzyme is characteristic of O alleles. Single nucleotide substitutions in exon 6 and 7 give rise to different ABO alleles.1
In our patient, the characteristic finding was weakened B expression with mixed field agglutination mimicking the B3 phenotype. Diminished expression of ABH antigens can occur with variant ABO alleles and in hematological disorders such as leukemia, myeloproliferative disorders, myelodysplastic syndrome and in some cases of Hodgkin's lymphoma. It has also been found in healthy elderly adults, pregnant females and neonates.2,5,6 Myeloid neoplasms have been reported to be more frequently associated with ABH antigenic alterations than lymphoid malignancies. AML associated with a weak A antigen was first elucidated by Van Loghem et al. in 1957 in a patient who had a previously normal A antigen on red cells. Flow cytometric analysis has shown decreased expression of ABH blood group antigens in patients with myeloid leukemia. Diminished expression of A and/or B antigens could be due to a blockage of the conversion of H substance to A and/or B substances or decreased synthesis of H antigens.7 Since the ABO locus encoding A and B transferases is in the 9q34 region, the chromosomal translocation (9 and 22) observed in patients with myeloid leukemia (both chronic myeloid leukemia and rarely AML) has also been linked to ABH red cell antigenic changes in these patients.2
Apart from the A3 and B3 alleles, mixed field reactivity can be encountered in recent transfusion, hematopoietic (bone marrow and stem cell) transplantation, fetomaternal hemorrhage, and twin or dispermic chimerism.8 Our patient had no such history. Mixed field reactions can also be observed in hematological malignancies (particularly myeloid leukemia) where a varying proportion of red blood cells do not agglutinate.2,7 Weakened expression of the A antigen on red cells mimicking the A3 phenotype was reported by Salmon et al. in patients with leukemia.5
The B3 phenotype is characterized by mixed field hemagglutination with anti-B and anti-A,B serum, absence of anti-B antigen in the serum and normal B antigen in the saliva (secretors). The B3 subgroup is the most frequent weak B phenotype.1 The B3 phenotype occurs with a frequency of 1/900 B and 1/1800 A1B in the Chinese population9 and with a frequency of 1:116,667 in French donors.10 Reports from India show the frequency of the weak B phenotype to be 1:86,687 among the donors of north India10 and 1:24,000 from Bombay.11 However the exact frequency is not known. Infrequent reporting of these findings could be due to lack of knowledge and the complex classification of subgroups.
B3 alleles arise due to amino acid substitutions (due to nucleotide substitutions in exon 7) in the glycosyltransferase B enzyme although a splice site mutation in exon 3 has been reported in only one B3 allele.5 There may be qualitative (difference in the ability to bind the antibody) as well as quantitative (number of antigens on red blood cells) differences in the B antigen of B3 red cells from normal B cells.9 It is safe to transfuse O group red cells in a patient with red blood cells expressing a variant ABO.12
Weakening of ABH antigen expression suggests a pre-leukemic state as they appear even before recognizing the malignancy. Follow-up of these patients will be helpful as the amount of antigens returns to normal on red blood cells with complete remission.5 Correct ABO grouping is required not only for ensuring a safe transfusion to patients but also for understanding the true epidemiology of weak subgroups in a given population.
ConclusionsWeak subgroups of the ABO blood group system are hardly reported in the day-to-day practice. Such ABO discrepancies are resolved by extended incubation of forward grouping tubes at 4°C, saliva secretor studies, adsorption-elution methods and pedigree analysis. Early recognition of the loss of ABH antigens is extremely important because it may indicate underlying sinistrous pre-leukemic states. Follow-up of the patients is necessary as these antigenic changes mirror the course of leukemia. In patients with hematological malignancies, correct ABO blood grouping is important as they require multiple transfusions and often undergo hematopoietic stem cell transplantation.
Conflicts of interestThe author declares no conflicts of interest.