Despite all the scientific progress that has been made on understanding the disease, prognosis for patients with relapsed and refractory Hodgkin's lymphoma remains poor and the treatment is palliative in the majority of the cases. Thus, the aim of this study was to present the results on the compassionate use of everolimus in a group of patients who were monitored at nine different centers in Brazil.
MethodsA 10-mg oral dose of everolimus was given to each patient daily. Response time was evaluated from the beginning of medication use until loss of response, toxicity or medical decision to cease treatment.
ResultsThirty-three patients were evaluated. The median age at the beginning of medication administration was 29 years. Patients had received a median of five prior therapies. Overall response rate was 45.4%, with 13 patients achieving partial response, two achieved clinical response, 14 remained with stable disease, two had disease progression, and two were not evaluated. Patients received a median of 14 cycles. Progression-free survival was nine months, and overall survival was estimated to be 36 months. Three patients used the medication for more than four years. The most frequently reported adverse events were thrombocytopenia and hypercholesterolemia. Three patients had pulmonary toxicity. Grade III and IV adverse events occurred in 39% of the patients.
ConclusionEverolimus was found to provide a response in a group of patients with refractory or relapsed Hodgkin's lymphoma who had adequate tolerability to the drug.
It is known that almost 80% of the patients with Hodgkin's lymphoma (HL) can be cured at first treatment. However, 10–15% will relapse and 5% of treated patients will not respond to the initial therapy and will become primary refractory. For these patients, the gold standard therapy is high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (HSCT). This second treatment can still rescue 50% of relapsed and refractory patients.1
Relapsed or refractory patients are a major concern and choosing the most suitable treatment for them remains a challenge. In these cases, despite all the progress that has been made in the last years, prognosis remains poor, and treatment will be palliative in the majority of cases.2,3 In addition, elderly patients who are not suitable for allogeneic HSCT because of the toxicity of the regimen, are a group for whom choosing the correct therapy is difficult.4
In August 2011, the anti-CD30 conjugated antibody, brentuximab vedotin, was the first drug approved for patients with relapsed and refractory disease after autologous HSCT. However, although this antibody showed good responses in chemo-refractory patients, with a 75% overall response rate (ORR – complete response/partial response), most patient responses end within six months, with only a few maintaining a response after 20 months.5 This treatment modality is generally very interesting as a bridge for allogeneic HSCT. Considering the same group of patients, another drug, anti-PD1 (nivolumab), was approved by the American Food and Drug Administration (FDA) recently after the results of a phase II study showed a very impressive ORR of 87%.
Another phase II study examined the use of everolimus in refractory and relapsed patients and showed an ORR of 47%, with very good and prolonged responses and few toxicities.6 Everolimus is a mechanistic target of the rapamycin (mTOR) inhibitor and is the focus of the current paper.
mTOR is a serine/threonine kinase involved in cell growth, metabolism and cell survival, regulation of the different stages of the cell cycle, and controlling the checkpoints that are responsible for the cell's responses to DNA damage.7–9 Furthermore, there is evidence that mTOR is an important component of angiogenesis, especially in the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt/mTOR activation pathway. This axis is directly associated with the vascular endothelial growth factor (VEGF), as its activation leads to the transcription of the HIF-1a pathway, which consequently increases VEGF levels.10
It is known that in HL there is an activation of the PI3K/Akt/TORC1 axis with consequent cell proliferation, angiogenesis, and tumor cell survival.10–12 One in vitro study demonstrated that everolimus acts on another pathway of HL tumor cells, namely, the CCAAT/enhancer binding protein beta (C/EBPb) pathway, decreasing activation of factor nuclear kappa B (NF-kB) and consequently inhibiting cell proliferation.13
Therefore, the aim of this study is to present the results on the compassionate use of everolimus in a group of patients who were monitored at nine different centers in Brazil.
Methods and patientsThis study is a retrospective analysis of refractory and relapsed HL patients enrolled in a Named Patient Program involving nine centers in Brazil. The first patient started the treatment in November 2010 and the last, in March 2015.
In order to be considered for the current study, patients were required to be fully eligible, that is, to be considered refractory/relapsed after autologous and/or allogeneic HSCT. The Eastern Cooperative Oncology Group (ECOG) score requested was <2. Patients were also required to be ≥18 years old, with an absolute neutrophil count (ANC) ≥1000×106/L, platelets ≥75,000×106/L, hemoglobin ≥8g/dL, serum creatinine ≤2× upper limit, serum total bilirubin ≤2× upper limit of normal (ULN) and aspartate aminotransferase (AST) ≤3× ULN. It was recommended that the medication should not be given to patients who had recently received radiotherapy (within four weeks) or immunosuppressive therapy (within three weeks), those who were using chronic systemic immunosuppressive agents, such as corticosteroids, had severe hemorrhagic diathesis or presented severe uncontrolled comorbidities (such as diabetes mellitus, infections, severe liver disease, lung disease with severe functional impairment). Moreover, female patients who were pregnant or were breastfeeding did not receive the medication.
The initial dose of 10mg/day was suggested by the manufacturer based on previous phase I and II studies. Decreasing the dose to 5mg/day or 5mg every other day was allowed when adverse events occurred.
Data were requested from the 13 centers that had had patients who participated in the Named Patient Program, but only nine centers sent the requested data.
The study was approved by the Ethics Committees of all participating centers (Hospital Santa Casa São Paulo, Hospital Santa Marcelina de São Paulo, Hospital das Clínicas de São Paulo – FMUSP, Hospital Israelita Albert Einstein, Hospital das Clínicas da UNICAMP, Hospital das Clínicas de Botucatu, Hospital das Clínicas da UFRS, Hospital do Câncer de Barretos and Hospital Santa Rita de Cássia). Informed consent was obtained from all patients included in the study.
An Excel spreadsheet was sent to all participating institutions, who were asked to provide the following data: patient's initials; gender; histologic subtype; date of birth; date of diagnosis; stage of the disease at diagnosis; international prognostic score (IPS) or early-stage risk factor (bulky mediastinal mass, 2 or more nodal sites, elevated erythrocyte sedimentation rate); treatment response; number of relapses; number of lines of treatment; date of autologous HSCT (if performed); date of allogeneic HSCT (if performed); date everolimus began being administered; best response observed; response assessment method [positron emission tomography–computed tomography (PET–CT) or computed tomography (CT)]; response duration; date everolimus administration was ceased; reason for treatment interruption; degree of toxicity; date of death or date of last contact.
Patients were eligible for assessment if they had received at least one cycle of treatment (28 days). Response assessment was not uniform at all centers. Most considered Cheson criteria14,15: complete remission was defined as the disappearance of all clinical and radiologic evidence of the disease; a partial response was defined as showing a greater than 50% reduction in the number of sites that were affected by the disease; and refractoriness was defined as not meeting the above criteria.
The time of response assessment was defined by the attending physician. At the reference center, Santa Casa São Paulo, assessments were conducted every four months with CT and/or PET–CT, depending on availability. Patient's medication was maintained if they showed a response or if their disease was stable. Because assessments were made at different centers, the criteria for interrupting treatment were individual and were mostly related to disease progression and toxicity. Some of the patients had nodal disease progression, but as they showed clinical response without constitutional symptoms, they were maintained under treatment until loss of clinical response.
Statistical analysisStandard descriptive analyses were carried out. The descriptive assessment of the stage of the disease comprised chemotherapy; first-line response; number of lines of treatment; type of HSCT performed; age at the beginning of everolimus administration; time between diagnosis and beginning of treatment with everolimus; response to treatment; duration of medication use; cause of treatment interruption; adverse events.
Overall survival was defined as the time between date of diagnosis and date of death or last follow-up. Progression free survival (PFS) was defined as the time between the date of initial diagnosis and the date of disease progression or death from any cause, whichever came first. The Kaplan–Meier estimator was used to examine survival curves, which were compared using the log rank test in the Statistical Package for the Social Sciences (SPSS) version 20.
The response rate was estimated as the ratio between the number of responses and the number of patients who were eligible for assessment. A bimodal 95% confidence interval (CI) was used to calculate overall response.
Toxicity was classified as grades I, II, III and IV for any event related to the use of medication.
ResultsData from the 33 patients with refractory and/or relapsed HL whose treatment with everolimus was initiated between November 2010 and March 2015 were evaluated (Table 1).
Patient characteristics (n=33).
| Characteristic | Number (%) |
|---|---|
| Stage at diagnosis | |
| Early | 3 (9%) |
| Advanced | 29 (87%) |
| NA | 1 (3%) |
| Gender (male) | 20 (60%) |
| Histologic type | |
| Classic | 33 (100%) |
| First-line chemotherapy | |
| ABVD | 31 (93%) |
| COPP | 1 (3%) |
| BEACOPP | 1 (3%) |
| Response to first treatment | |
| CR | 19 (57.5%) |
| PR | 7 (21.2%) |
| Refractory | 7 (21.2%) |
| Number of lines of treatment | |
| Median | 5 |
| Range | 3–7 |
| Time between diagnosis until initial everolimus administration (months) | |
| Median | 59 |
| Range | 14–111 |
| Previous HSCT | |
| None | 2 (6%) |
| Autologous | 27 (81.8%) |
| Allogeneic | 2 (6%) |
| Both | 2 (6%) |
| Age at the time of initial everolimus administration | |
| Median | 29 |
| Range | 20–70 |
| Best everolimus response | |
| CR | 2 (6%) |
| PR | 13 (39.4%) |
| SD | 14 (42.4%) |
| Progression | 2 (6%) |
| NA | 2 (6%) |
NA: not assessable; HSCT: hematopoietic stem cell transplantation; ABVD: adryamicin, bleomycin, vinblastine and dacarbazine; COPP: cyclophosphamide, oncovin, procarbazine, prednisone; BEACOPP: bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, prednisone; CR: complete response; PR: partial response; SD: stable disease.
When assessing the number of lines of treatment that had previously been used by the patient, the average coincided with the median, five chemotherapy and/or radiotherapy regimens (range: 3–7).
Two of the patients had not been submitted to HSCT prior to receiving everolimus; twenty-seven had undergone autologous HSCT alone, two had undergone allogeneic HSCT alone (one due to mobilization failure, and one with no data) and two patients had undergone autologous and allogeneic HSCT.
The median time between diagnosis and the beginning of treatment with everolimus was 1652 days (54 months; range 14–111 months).
The assessments were conducted using CT in 16 patients, PET–CT in nine patients, a combination of CT and PET–CT in five patients and physical examination was the only assessment employed in the evaluation of one patient.
Regarding the evaluation of patients’ response to everolimus, two of the patients achieved a complete response (6%), 13 had a partial response (39.4%), and the disease remained stable in fourteen (42%). Two patients had progression of the disease (6%), and two (6%) had no documented evaluation because they progressed before the first image could be taken. Considering partial and complete responses as an overall response, 45% [15/33; 95% CI: 28.5–62.4%] responded to the treatment with everolimus.
Median treatment time in this cohort was 317 days (10 months). Thirteen patients underwent treatment for over one year, and three patients had been receiving treatment for more than four years.
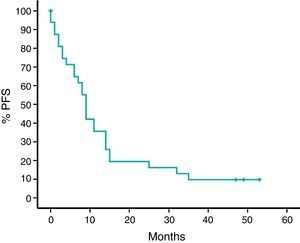
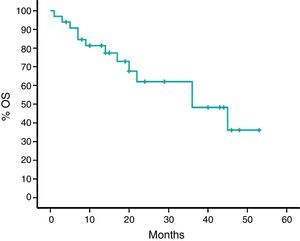
With a median follow-up time of 24 months, median survival rate was 62%, and the estimated median survival rate was 36 months. PFS was nine months (95% CI: 7–10) (Figures 1 and 2).
The adverse events reported were grade I and II mucositis in one patient (3%); grade III and IV mucositis in one (3%); itching reported only at the beginning of medication use in one (3%); grade I skin rash in two (6%); oral ulcers in one (3%); intestinal ulcers in one (3%); reactivation of cytomegalovirus (CMV) in one (3%); emergence of pleural effusion in one (3%); adynamia in two (6%); anasarca in one (3%); altered kidney function in one (3%); and pulmonary toxicity with the onset of coughing, wheezing and pulmonary infiltrates in five (15.1%) (Table 2). Hypercholesterolemia was described in five (15.1%) patients, some of whom required lipid-lowering agents. With regard to hematologic toxicity, five (15.1%) had grade I and II thrombocytopenia, and three (9%) had grade III and IV thrombocytopenia. Grade I and II neutropenia was reported in one patient (3%), and grade III and IV in three (9%). One patient (3%) had a diagnosis of acute myeloid leukemia (AML) while undergoing treatment with everolimus; this patient had been using everolimus for 13 months and had already undergone six lines of treatment, including HSCT. One patient (3%) was diagnosed with pulmonary tuberculosis while using the medication.
Adverse events in the Brazilian cohort.
| Grades I and II | Grades III and IV | |
|---|---|---|
| Mucositis | 1 (3%) | 1 (3%) |
| Itching | 1 (3%) | |
| Rash | 2 (6%) | |
| GIT ulcers (oral and intestinal) | 2 (6%) | |
| Reactivation of CMV | 1 (3%) | |
| Pleural effusion | 1 (3%) | |
| Adynamia | 1 (3%) | 2 (6%) |
| Anasarca | 1 (3%) | |
| Renal function alteration | 1 (3%) | |
| Pulmonary toxicity | 4 (12%) | 1 (3%) |
| Hypercholesterolemia | 5 (15%) | |
| Thrombocytopenia | 5 (15%) | 3 (9%) |
| Neutropenia | 1 (3%) | 3 (9%) |
GIT: Gastrointestinal tract; CMV: Cytomegalovirus.
In three (9%) patients medication was discontinued due to death (not reported whether by progression or toxicity), toxicity was the reason for interruption in five (15.1%) patients and progression in 17 (51.5%). Treatment for one patient was discontinued due to the diagnosis of AML, and another had a very good partial response and was referred for allogeneic transplantation. Five patients remained in the program and were still receiving the medication while this analysis was being conducted.
DiscussionPatients with primary refractory HL who are not allergenic HSCT candidates, or in whom the disease relapses following this treatment modality, present a very poor prognosis.1 New drugs and their incorporation in the treatment are essential to increase response and survival rates in this specific group of patients. One example is mTOR inhibitors with their rationale use in HL being based on studies that demonstrate activation of the PI3K pathway in this type of tumor.7
This study is not a clinical trial but a retrospective study on the use of an oral experimental medication in a group of previously treated patients who had few alternatives and the chance of a potential increase in survival. It is important to emphasize that, by the time everolimus was available for compassionate use, brentuximab vedotin (anti-CD30) was not approved for use in Brazil; thus, there were few therapeutic options for relapsed and refractory HL patients. At that time, only one patient had imported and used brentuximab vedotin and had suffered disease progression within a short time.
Among the study's limitations are its retrospective data collection and that it has neither standardized nor uniform assessments of disease response. Another limitation is its lack of formal documentation of required information on adverse events. Therefore, we consider adverse events could be underreported, especially grade I and II events. Nevertheless, the most severe cases, in which treatment was reduced or interrupted, have been fully reported.
Another limitation was the inability to standardize the range and criteria for evaluating patients. Because these analyses were carried out at the centers responsible for the patients’ treatment, some of the data among the more objective responses may have been overestimated. Two of the patients had nodal progression, but their clinical response was maintained. Therefore, they remained in the program until loss of clinical response was observed. One of those patients was still using the medication four years after starting to receive it.
It is important to highlight that the continuous use of the medication, despite nodal progression, is considered to be appropriate a posteriori, given the impossibility of new treatment modalities at that moment and the benefit to the patient, who remained clinically well and whose disease progression was very slow.
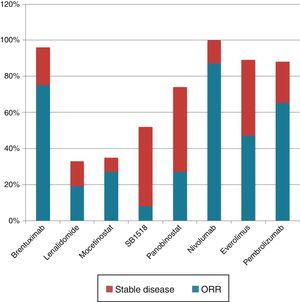
There are few published studies on the use of this medication in this population. More precisely, there is one phase II study carried out in the USA that assessed 19 patients, of whom only 16 used everolimus for more than one cycle (28 days of treatment).6 In this US cohort the ORR was 47% (9/19; 95% CI: 24–71%), with eight (42%) patients showing partial response and one (5.2%) patient having complete response. In the Brazilian cohort, the ORR was 45.4%, with 13 (39.3%) patients achieving partial response and two (6%), complete response. In addition, two updates of the US phase II study published as conference abstracts demonstrated that, with greater numbers of patients (38 and 57), response rates were only 37% of 38 and 42% of 57, with 34% of 38 and 33.3% of 57 patients showing partial response, and 2.6% of 38 and 8.8% of 57 showing complete response (Figure 3).13,14
Only the US phase II study6 can be used for further analysis and comparison of data given that results of its updated versions, with 38 and 57 patients, respectively, have not yet been fully published.16 In the US study, the median time between HL diagnosis and the beginning of everolimus administration was 3.5 years; while in the Brazilian cohort it was 4.5 years. The median time between the beginning of everolimus use and response was 3.6 months in the US study. Unfortunately, we could not perform this analysis due to lack of objective data in the Brazilian cohort. Also in the US study, response duration time was 7.1 months (95% CI: 3.9–14.8) with four patients using the medication for over 12 months with no progression. In the Brazilian cohort, PFS was nine months (CI 95%: 7–10) with a median treatment duration of ten months, while in the US study PFS was 6.2 months (CI 95%: 5.9–9.5).
The long treatment duration may be explained by the fact, mentioned above, that there was a group of patients maintained in the program even after nodal disease progression. It seems these patients might still have clinical benefits from the treatment for not showing signs of constitutional symptoms. On retrospectively reviewing this conduct, it could be considered appropriate, as these patients have been receiving the medication for over four years, with excellent quality of life and very slow nodal progression. It is worth mentioning that such a long response time could be associated with the fact that three patients have continued using the medication for over four years, which shows its good tolerability and safety.
Overall survival was estimated in the US study at 25.2 months (95% CI: 13-not yet available; median follow-up time was 24 months) versus 36 months (95% CI: 15–56) in the Brazilian cohort (the median follow-up was also 24 months).
In the US study, treatment discontinuation had the following reasons: progression in 16 (84%) patients; pneumonia in one (5%); and infection leading to death in one (5%). In its updated version, progression is described as the reason for discontinuation in 11 (29%) patients.6
In the Brazilian cohort, progression of the disease was the reason for discontinuation in 17 (51%) patients, while toxicity was the reason in six (18%) and one (3%) patient was diagnosed with AML; one was referred to allogeneic HSCT following a good response with the medication. In this present cohort, three (9%) patients reportedly interrupted treatment due to death without further specifications.
As mentioned, the report of adverse events in the Brazilian cohort is probably underestimated because the data were collected retrospectively and were not standardized. In this cohort, 13 (39%) grade III and IV events were reported, whereas the US study reported 74% of grade III and IV events, and 40% of 38 and 57.9% of 57 events after it was updated with increased numbers of participants. Fifty-three percent of the US study patients had their dose decreased due to adverse events but, unfortunately, we could not collect this data from the Brazilian cohort. Pulmonary toxicity in a cohort with refractory disease is difficult to attribute solely to the studied drug, as patients had usually taken more than one pulmonary toxic drug. As for hypercholesterolemia, most patients did not need any treatment, and in the three patients who used statins, cholesterol became normalized.
Another significant piece of data was that only a few patients in the Brazilian cohort were referred to allogeneic HSCT after exhibiting responses elicited by everolimus. This decision was made by each center with the underlying reasons being the high tolerability to everolimus association with the patients’ quality of life versus the high risk related to allogeneic HSCT as was corroborated by the recent approval of everolimus in Brazil, the inappropriate infrastructure to perform this procedure at centers and patients’ refusal to undergo HSCT. No data on this concern were published either in the US phase II study or in the updated results.
Everolimus is still under study and combinations of other antineoplastic agents have been tested. A phase I study with everolimus associated with panobinostat had a good ORR (43%), albeit with high toxicity.16 Other combinations are being tested such as the combination of everolimus and lenalidomide, and the combination of sorafenib and histone deacetylase inhibitors.17
In comparison with other drugs used for refractory patients, everolimus has less response compared to brentuximab vedotin (79%),5 pembrulizumab (65%),18 and nivolumab (87%),19 but its response is higher than those of SB1518,20 lenalidomide,21 mocetinostat22 and panobinostat.23 It is evident that there is a group of patients who may benefit from using this medication, while there is another group that either does not show any response or whose response to the drug lasts only briefly. Identifying the subgroup of responding patients would have a significant impact on optimizing medication use with respect to response rates and significant and permanent adverse events and costs.
In conclusion, we believe that this study is relevant even if its methodology is retrospective. Everolimus is still an experimental medication and has been proven to be active and improve the results in a group of selected patients in which there are few therapeutic possibilities, such as those with refractory and relapsed HL. Furthermore, because of the good responses observed in a subgroup of patients, these results allow the study of this medication compared to other medications that have different mechanisms of action.
Compliance with ethical standardsThe institutional review board of each participating institution approved this study design, and this study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before their inclusion in the study.
Conflicts of interestThe authors declare no conflicts of interest.