Diagnosis and treatment of iron deficiency anemia in older subjects improves their quality of life. Serum ferritin as a marker of iron stores is an acute phase protein. In older subjects who usually have many concomitant chronic medical conditions, serum ferritin may increase in response to inflammatory processes irrespective of iron stores. This study was performed to determine the diagnostic properties of serum ferritin in the diagnosis of iron deficiency anemia in older subjects.
MethodsThis case–control study included all the inhabitants of Amirkola town who participated in the Amirkola Health and Aging Project. Diagnosis of anemia was confirmed based on a hemoglobin level <13g/dL in men and <12g/dL in women and iron deficiency anemia by percent transferrin saturation <15%. A receiver operating characteristic curve was constructed to determine an optimal serum ferritin cutoff value to differentiate patients with and without iron deficiency anemia at the highest sensitivity and specificity.
ResultsEighty patients with iron deficiency anemia and 160 cases of anemia without iron deficiency (mean age: 72.9±8 and 71.6±7.6 years, respectively; p-value=0.37) were analyzed. In iron deficiency anemia, the mean serum ferritin was significantly lower (p-value=0.036) compared to patients without iron deficiency anemia. Serum ferritin with a cutoff level of 100ng/mL differentiated patients with and without iron deficiency anemia with a sensitivity of 60% and specificity of 59% and area under the receiver operating characteristic curve of 0.615±0.040 (95% confidence interval: 0.536–0.694; p-value=0.004).
ConclusionThese findings indicate that in elderly subjects, iron deficiency anemia may develop with higher levels of serum ferritin. Hence, the conventional cutoff of serum ferritin for the diagnosis of iron deficiency anemia in young adults is not appropriate for the elderly population.
Anemia affects one third of the world population with nearly half of the patients with anemia suffering from iron deficiency.1
In elderly people, even low levels of anemia affect the quality of life and increase the risk of mortality resulting in many medical conditions such as cardiovascular and cognitive disorders, osteopenia, muscle weakness, falls and fractures, and depression.2 Several factors such as inflammatory processes, chronic renal failure, and gastrointestinal and nutritional disorders are associated with anemia, in particular iron deficiency anemia (IDA). In the aged, the prevalence of anemia increases with age and ranges from 8% to 25%, nonetheless the causes of anemia, including the diagnosis of the iron deficiency state, remain undetermined in many patients.3–6 Currently, serum ferritin (SF) (ranging from 40 to 200ng/mL) is a measure of iron stores in healthy adults. However, its diagnostic capability varies across different studies with regard to cutoff points.7–9
In elderly populations, changes in SF concentrations do not always correlate with variations in iron stores because ferritin is an acute phase protein and is affected by inflammatory processes irrespective of the iron store status.
Many chronic medical conditions in the general population such as obesity, metabolic syndrome, chronic obstructive pulmonary disease and diabetes are also prevalent in the elderly.10–19 These conditions are usually associated with inflammation.10,11,20,21 Coexistence of these comorbidities in aged people may be associated with elevated levels of acute phase proteins including SF. This situation results in the development of functional IDA (anemia of inflammation) which is associated with disproportionate release of iron from iron stores to compensate body demands with subsequent IDA. In these cases, in contrast to absolute IDA, SF does not reduce concomitantly with iron stores.
This issue creates difficulties in the diagnostic capability of the ferritin test to diagnose IDA. Thus, the classic cutoff value of SF as applied to young adults may be inappropriate for the diagnosis of IDA in the elderly. These observations warrant further investigations to determine a SF level with more reliable diagnostic properties. A systematic review of 55 studies found variations in SF test results across populations with and without inflammatory processes, liver disease or neoplastic diseases. Although ferritin is not an excellent measure of iron stores, it is a practical and widely used method to assess iron stores.22
For these reasons, the present study was designed to determine an optimal SF level to differentiate elderly patients with and without IDA with greater sensitivity and specificity. The secondary purpose of this study was to determine the diagnostic properties of different levels of SF in the diagnosis of IDA in a cohort of the elderly subjects aged 60 years and older.
MethodsThe patients of this case–control study were recruited among the participants of the Amirkola Health and Aging Project (AHAP). This project was carried out in Amirkola, Babol, a town located near the Caspian Sea, northern Iran. The project was funded by the Vice-Chancellery of Research and Technology, Babol University of Medical Sciences for the investigation of geriatric medical problems such as falling, bone fragility and fractures, cognitive impairment and dementia, poor mobility and functional dependence. The baseline stage of this project was carried out in 2011 and 2012. All inhabitants aged 60 years and over were invited to participate in this study with 72.3% of the invited subjects participating.12 All patients with anemia, defined as a hemoglobin level lower than 13g/dL in men and 12g/dL in women, were included in the study. Participants with a history of transfusion within six months prior to the start of this study, those taking iron supplements, and patients with chronic renal failure and on maintenance hemodialysis were excluded.
All patients gave informed consent and the proposal of this study was approved by the Ethics Committee of the Babol University of Medical Sciences, Babol, Iran.
Data were collected regarding serum iron, percent of transferrin saturation and SF and the prevalence of coexistent chronic medical conditions was recorded.
Data regarding chronic diseases were provided by clinical examination, interviews and review of medical records. The diagnosis of IDA was confirmed based on transferrin saturation levels of less than 15%. Details of patient selection, data collection and laboratory test results have been described elsewhere.12
In the statistical analysis, the participants of this study were classified as patients with and without IDA. Eighty patients with IDA were compared with 160 patients with anemia without iron deficiency. The two groups were compared regarding SF and percent of transferrin saturation.
The diagnostic ability of SF was determined by receiver operating characteristic (ROC) curve analysis. The optimal cutoff levels that differentiated patients with and without IDA at the highest sensitivity and specificity rates were determined using Youden's index calculated by sensitivity+specificity–1. The accuracy of test was assessed based on area under the ROC curve (AUC). The distribution of all variables was tested for normality using the Kolmogorov–Smirnov test. Parametric and non-parametric tests were used for analyses of variables with and without normal distribution, respectively. The Statistical Package for the Social Sciences (SPSS) software version 18 was employed for analysis.
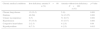
ResultsThe Amirkola Cohort Profile included all inhabitants living in the 34 districts of Amirkola town, 2234 of whom were aged 60 years or older when this study began (1158 men and 1076 women). Thirty-five out of 2234 participants were excluded and 114 women and 126 men out of 1994 participants (12.03%) who had anemia were studied. Eighty patients with IDA, with a mean age of 72.9±8 and 160 patients without IDA with a mean age of 71.6±7.6 years old (p-value=0.37) were analyzed (Table 1).
Comparison of iron parameters in elderly subjects aged 60 years and older with and without iron deficiency anemia.
| Variables | IDA present n=80 | IDA absenta n=160 | p-Valuec |
|---|---|---|---|
| Age (Mean±SD) – years | 72.9±8 | 71.6±7.6 | 0.37 |
| Hemoglobin level – g/dL | 9.7±1.3 | 10.1±1.2 | 0.018 |
| Serum iron – μg/dL | 35.8±8.3 | 95.3±26.4 | 0.001 |
| Transferrin – μg/dL | 333.1±26.7 | 269±28.8 | 0.001 |
| Transferrin saturation level – mean, % | 10.85±2.2 | 31.1±8.2 | 0.001 |
| Serum ferritin – ng/mL | 148.5±147.7 | 188.4±134 | 0.036 |
IDA: iron deficiency anemia.
Coexistent common chronic medical diseases such as, hypertension, urinary incompetence, diabetes, chronic lung disease, hypothyroidism and congestive heart failure were found in 41.6%, 24.5%, 34.1%, 7.5%, 4.1% and 2.5% of the total patients with anemia, respectively. Prevalence of comorbidities in patients with and without IDA are presented in Table 2. The prevalence of diabetes, chronic lung disease and hypothyroidism was significantly higher in patients with IDA but the prevalences of incontinency and hypertension were lower compared to those without IDA.
Prevalence of chronic medical conditions in patients with and without iron deficiency anemia in elderly subjects aged 60 years and more.
| Chronic medical conditions | Iron deficiency anemia N=80 n (%) | Anemia without iron deficiency N=160 n (%) | p-Value |
|---|---|---|---|
| Chronic lung disease | 13 (16.2) | 5 (8) | 0.001 |
| Diabetes | 48 (60) | 34 (21.25) | 0.001 |
| Urinary incompetence | 8 (5) | 51 (63.7) | 0.001 |
| Hypertension | 57 (35.6) | 43 (53.7) | 0.001 |
| Congestive heart failure | 2 (2.5) | 4 (2.5) | 0.64 |
| Hypothyroidism | 6 (7.5) | 4 (2.5) | 0.013 |
In iron deficient patients, serum iron concentration was significantly lower, serum transferrin was significantly higher, and both transferrin saturation and SF were significantly lower compared with patients without iron deficiency (Table 1).
SF at cutoff level of 100ng/mL differentiated patients with and without IDA with a sensitivity of 60% and specificity of 59% with the area under the ROC curve of 0.615±0.040 [95% Confidence interval (95% CI): 0.536–0.694; p-value=0.004].
This cutoff level yielded positive and negative predictive values of 45% and 74%, respectively. Further analyses were performed to determine the diagnostic performance for other levels of SF (Table 3). SF levels lower than 18ng/mL yielded the highest likelihood ratio of 3.71 (95% CI: 1.5–8.4), sensitivity of 16% and specificity of 96% for the diagnosis of IDA (Table 3). Serum cutoff levels of 45ng/mL and 60ng/mL yielded positive likelihood ratios of 2.54 (95% CI: 1.4–4.4) and 2.33 (95% CI: 1.4–3.73), respectively. In patients with SF levels less than 100ng/mL, the likelihood of IDA decreased to 1.61 (95% CI: 1.18–2.2) with negative predictive value of 72% and likelihood negative ratio of 0.76.
Diagnostic properties for different cutoff levels of serum ferritin in diagnosis of iron deficiency anemia in elderly patients aged >60 years old.
| 18ng/mL | 45ng/mL | 60ng/mL | 100ng/mL | |
|---|---|---|---|---|
| Sensitivity (95% CI) | 16 (8–24) | 29 (19–30) | 35 (25–45) | 51 (40–62) |
| Specificity (95% CI) | 96 (92–99) | 89 (84–94) | 85 (79–91) | 68 (61–75) |
| PPV (95% CI) | 66 (44–86) | 56 (41–71) | 53 (40–67) | 44.3 (34–55) |
| NPV (95% CI) | 69 (63–70) | 71 (65–78) | 72 (66–79) | 73.4 (67–81) |
| LR+ (95% CI) | 4 (1.5–8.4) | 2.64 (1.4–4.4) | 2.3 (1.4.3.75) | 1.6 (1.18–2.20) |
| LR− (95% CI) | 0.87 (0.79–0.97) | 0.8 (0.69–0.93) | 0.76 (0.64–0.91) | 0.72 (0.56–0.92) |
95% CI: 95% confidence interval; PPV: positive predictive value; NPV: negative predictive value; LR+: likelihood ratio positive; LR−: likelihood ratio negative.
The results of this study indicate that, in elderly subjects, SF tests have a different diagnostic ability according to the cutoff levels. In the present study, the optimal SF with a cutoff point of 100ng/mL yielded the highest sensitivity and specificity for the diagnosis of IDA in subjects aged 60 years and older. Based on this study, SF of less than 100ng/mL identified 51% of patients who had transferrin saturation <15%, whereas SF >100ng/mL identified 74% of subjects without IDA indicating a higher negative predictive value compared to the positive predictive value. This suggests that in elderly subjects SF levels >100ng/mL compared with <100ng/mL yield greater ability to exclude rather than confirm IDA.
This study found different diagnostic properties across various SF levels. Compared to the cutoff level of 100ng/mL, cutoffs of 18ng/mL, 45ng/mL and 60ng/mL yielded greater positive predictive values as well as positive likelihood ratios but lower sensitivity for the diagnosis of IDA (Table 3).
On the other hand, while serum cutoff levels of 45 and 60ng/mL yielded comparable diagnostic properties, levels ≤18ng/mL compared with other cutoff points exhibited higher specificity and likelihood ratio but lower sensitivity.
Overall, by increasing SF cutoff levels, the sensitivity and the negative predictive value of this test in the diagnosis of IDA increases at the expense of decreasing specificity and the positive predictive value.
In the present study, the mean SF in patients with IDA was higher than 100ng/mL, whereas in a study of apparently healthy 80-year-old Danish men and women, the median SF value was 100ng/mL in men and 78ng/mL in women. In 9% of these subjects, the SF levels were >300ng/mL.23 In a study of 73 patients with anemia and chronic diseases by Coenen et al., SF concentrations of less than 70ng/mL were always indicative of IDA.24 In patients with inflammatory disease such as rheumatoid arthritis, iron deficiency anemia may develop at higher levels of SF and so the cutoff point is expected to be higher.25 The results of a systematic review suggest that further investigations are needed on the diagnosis of IDA in conditions with SF concentrations lower than 100ng/mL.26
The results of another study of anemic veterans with a wide variety of general medical comorbidities were partly similar to this study. The study found a sensitivity of 64.9% with SF ≤100ng/mL and a specificity of 96.1% to detect patients with IDA.27
The cutoff points for SF in patients with IDA in previous studies vary from 12 to 100ng/mL.7,23,24,27 In a randomly selected sample of 38-year-old women, SF <16ng/mL was the best cutoff level to differentiate patients with and without iron deficiency with a sensitivity of 75% and specificity of 98%; the iron stores began to disappear at SF levels from 25 to 40ng/mL.7 However, compared to the current study the age of patients was lower.
In another study of elderly patients, SF measurement was the best diagnostic test to discriminate patients with and without IDA. In this study, the likelihood of diagnosis of IDA in cases with SF from 18 to 45ng/mL was 3.12 and in those with less than 18ng/mL, it was 41.47, with a negative predictive value of 72%.22
This study indicates that, in elderly people, SF has less diagnostic ability compared to percent of transferrin saturation. In a study of 49 consecutive subjects aged 80 years or more with IDA as confirmed by bone marrow aspiration, correct diagnosis by SF, serum iron and percent of transferrin saturation was possible in only 16.3%.28
Variations in the diagnostic ability of SF to diagnose IDA across various studies may be attributed to factors such as the diagnostic criteria applied for IDA, characteristics of the study patients, and the prevalence of comorbidities in the study patients.6,7,22,24–32 The presence of comorbidities in the study population, particularly in older subjects, is associated with elevated levels of acute phase proteins including ferritin and thus may affect the cutoff level and change the results.
In a study of patients in the general practice aged 65 years and older, 23% suffered from at least one chronic disease with 15% suffering from more than one chronic disease such osteoarthritis, diabetes, chronic obstructive pulmonary disease, coronary artery disease, hypertension and diabetes.18 In another study, 82% of aged Medicare beneficiaries had one or more chronic conditions and 65% had multiple chronic conditions.17 About 82% of the participants aged 65–84 years of the 2003 National Sleep Foundation study reported one or more of 11 medical conditions and nearly 25% of respondents had four or more conditions such as obesity, arthritis, diabetes, lung disease, stroke and osteoporosis.19 In the present study hypertension, urinary incontinency, diabetes and chronic lung disease were found in significant proportions of patients in both groups. Most chronic medical conditions particularly diabetes, urinary incontinency and chronic lung disease are associated with inflammatory processes.10,11,13–16,20,21 The prevalence of both general and abdominal obesity increases with aging.13,14 Obesity is associated with inflammation and there is a positive correlation between the body mass index and SF.33
The limitations of this study should be considered. One major limitation is the lack of bone marrow aspiration for definitive diagnosis of iron deficiency anemia. Although absence of iron in the bone marrow is considered the gold standard diagnostic test for diagnosis of IDA, the lack of iron stores in the bone marrow aspirate is not necessarily predictive of IDA.34 In a retrospective study of 12 patients with depleted iron stores, iron deficiency was the cause of anemia only in 50% of the patients.34 However, Klantar-Zadeh et al. reported high sensitivity and specificity of the percent of transferrin saturation test in the diagnosis of IDA in chronic renal disease with inflammatory conditions.35 Another limitation is related to lack of data concerning the assessment of serum C-reactive protein and other measures of inflammation to show the existence of inflammatory processes. However, the high prevalence of diabetes, chronic lung disease and urinary incontinence even in the control group indicates that there were chronic comorbidities in the elderly people, and consequently inflammatory processes are common.
The strength of this study is related to the study sample which included all participants of the Almirkola Cohort Study that enrolled all the inhabitants of Amirkola, a small town in northern Iran. Another strength is related to the homogeneity of the study population in respect to demographic features, lifestyle and ethnicity.
The clinical significance of these findings is related to the incapability of the SF test as a measure to identify IDA in elderly people. These findings suggest that SF levels in many elderly subjects with IDA may be normal or higher than normal, and thus SF using conventional cutoff levels is not a reliable measure in the diagnosis of absolute IDA.
ConclusionIn conclusion, the results of this study indicate that SF is not an appropriate test for the diagnosis of IDA in the elderly subjects aged 60 years and older. Based on these findings the SF cutoff level of 100ng/mL can predict half of the patients with absolute IDA, whereas SF >100 yields a 74% negative predictive value, indicating that in elderly people higher levels of SF are better to exclude IDA rather than low levels of SF to confirm IDA. This context requires further studies.
Conflict of interestThe authors declare no conflicts of interest.
We thank the Clinical Research Development Unit Of Rouhani Hospital for assistance in manuscript submission and references arrangement.