Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoPostoperative anemia is a complex clinical issue that requires attention due to its ramifications on the patient's recovery and prognosis. Originating from multiple determinants, such as intraoperative blood loss, hemolysis, nutritional deficiencies, systemic inflammation and impact on the bone marrow, postoperative anemia has varied and often challenging presentations. Patients undergoing major surgical procedures, in particular, are susceptible to developing anemia due to the considerable associated blood loss. Accurate diagnosis plays a crucial role in the approach, requiring meticulous hematological analysis, including hemoglobin, hematocrit and reticulocyte count, as well as an in-depth investigation of the underlying causes. An additional challenge arises in the form of the excessive practice of phlebotomy during hospitalization for clinical monitoring. Although it is essential to assess the progression of anemia, frequent removal of blood may contribute to iatrogenic anemia, further delaying recovery and possibly increasing susceptibility to infection.
Although most guidelines and recommendations focus on the issue of preoperative anemia, the occurrence of this complication in the postoperative period is equally important, and can affect up to 80% of patients.1 Adequate diagnosis and pharmacological management of postoperative anemia should also be discussed and implemented within Patient Blood Management (PBM) programs with the aim of accelerating patient recovery, reducing transfusion demand and improving clinical outcomes.
The etiology of postoperative anemia tends to be multifactorial and may result from unidentified and worsened preoperative anemia or iron deficiency, decreased erythropoiesis due to inflammation associated with surgical trauma, blood loss and coagulopathy secondary to the procedure, excessive phlebotomies for laboratory tests and excessive normovolemic hemodilution during the surgical procedure.2
Furthermore, the prevalence of anemia increases in patients with a greater number of comorbidities, such as heart disease and renal failure, and these are precisely the patients who are more likely to undergo surgeries with a greater potential for bleeding. Therefore, the lack of adequate treatment of anemia in the postoperative period leads to a greater possibility of blood transfusion, since this group of patients will have less tolerance to anemia due to their comorbidities.3 Therefore, it is essential to identify and intervene in the main modifiable risk factors for postoperative anemia, as will be discussed below.
Postoperative anemia: risk factors and diagnosisIt is important that the entire team that cares for patients who have recently undergone any surgical procedure is aware of risk factors that may precipitate or worsen the evolution of postoperative anemia. Among them, we can list: advanced age, female sex, smaller body surface, existence of anemia already identified pre-operatively, occurrence of blood loss (due to surgical bleeding, the occurrence of coagulopathy secondary to the procedure and phlebotomies performed during hospitalization), as well as the magnitude of inflammation generated by surgical trauma leading to a decrease in erythropoiesis.4
Another factor that must be evaluated is the excessive fluids administered during surgery, which can lead to hemodilution, causing dilutional anemia or worsening pre-existing anemia. Other nutritional deficiencies identified preoperatively, such as vitamin B12 and folate deficiency, must be remembered and it is essential to ensure that adequate treatment is maintained. Finally, low nutritional intake and the use of medications are very common at this stage and can also contribute to bone marrow hypoproliferation.2
In general, preventive measures should be encouraged institutionally throughout the entire preoperative period. These include identification and treatment of preoperative anemia; minimization of preoperative blood loss, which must be carried out using medications such as antifibrinolytics, minimally invasive surgical techniques, optimization of laboratory test collections, as well as intraoperative blood recapture, when possible; and the prevention of excessive hemodilution.
Hemoglobin dosage is then recommended postoperatively for all patients who:
- i)
underwent major surgeries, defined as those in which there is blood loss of more than 500 mL or duration of more than two hours,
- ii)
who had anemia preoperatively or
- iii)
who had moderate or severe bleeding during the procedure, regardless of its size.5
Furthermore, hemoglobin levels should be monitored in the first few days after surgery under these same conditions, remembering the possibility that they may decrease compared to the immediate postoperative period.
In most cases of uncomplicated postoperative recovery, a nadir in hemoglobin concentration is expected within the first three to four days after surgery; but, in patients with complications or prolonged hospitalization, this monitoring must be extended.2 In this specific scenario, ideally, there should be intensive clinical monitoring, with vital signs every hour in the first six hours after the procedure and laboratory monitoring on the 1st and 3rd postoperative days, but this should be intensified according to the clinical changes identified and the patient's evolution. Accordingly, for patients with complications, this monitoring period tends to be longer.2
The diagnosis of iron deficiency in the postoperative period is more difficult, because ferritin levels may be elevated as part of the inflammatory response in the acute phase after surgery.5 A ferritin concentration <100 mg/dL in the first 24 h after surgery (a phase in which there is not any noticeable increase due to inflammation yet) indicates insufficient iron reserves to sustain erythropoiesis with significant potential for hemoglobin decline in the postoperative period.6
In general, in the postoperative period, iron deficiency should be defined by a ferritin concentration <100 ng/mL or ferritin 100-300 ng/mL and transferrin saturation <20%, or hemoglobin content of reticulocytes <28 pgs. Large blood losses are also indicative of the need for iron replacement in anemic post-surgery patients, even without assessment of available iron status.2
Optimized management of postoperative anemiaIt is important to highlight that blood loss does not necessarily end with the end of surgery and that the postoperative period is also a critical phase for the occurrence or worsening of anemia. Therefore, instituting appropriate treatment will allow for better patient recovery and higher hemoglobin levels at hospital discharge, thereby favoring rehabilitation.1-3
The choice of treatment depends on the severity and etiology of the anemia, the patient's comorbidities and the presence of surgical complications.2 Iron supplementation should be considered in patients with iron deficiency or a significant drop in hemoglobin in the immediate postoperative period, if there are no major complications. However, there are no studies that identify the ideal period for this use.5 In the postoperative period, when the administration of iron is necessary, the intravenous route with a high concentration of iron in a single dose should preferably be used, considering the greater probability of effective results in a short period of time, as long as there are no contraindications.5 Anyhow, in this case, the remaining period of hospitalization should be seen as an important opportunity to replace iron and to reduce the possibility of anemia at hospital discharge or even progression to acute complications in which transfusion ends up being the only available option.
Iron supplementation starting early in the postoperative period should be considered in patients with iron deficiency or a significant reduction in postoperative hemoglobin (<11 g/dL).3 The Ganzoni formula can be used to calculate the iron deficit in this period, in a more individualized way, and the dose of parenteral iron to be replaced, considering the target hemoglobin level of 13 g/dL and the presumed iron reserve of 500 mg:1,6
The choice of treatment for postoperative anemia will be based on the type of anemia, degree of severity, patient comorbidities and the presence of complications related to surgery.
Oral iron replacement can be used, but the inflammatory response induced by surgery stimulates the synthesis and release of hepcidin, which, in high concentrations, inhibits intestinal iron absorption. Therefore, the use of oral replacement may be ineffective at this stage. Consequently, in the presence of anemia associated with inflammation, the use of intravenous iron is preferably recommended.6
Patients with Hb <10 g/dL or with evidence of postoperative iron deficiency (ferritin <100 ng/mL or ferritin <300 ng/mL and transferrin saturation <20% in the immediate postoperative period) should preferably receive intravenous iron instead of oral iron due to the greater effectiveness of parenteral replacement. Recent studies have demonstrated better results with the use of high-dose formulations, with an increase in hemoglobin and a reduction in the need for transfusion, without serious adverse effects.7,8
The use of iron intravenously is well tolerated and does not increase the risk of infection; with the exception that, in the case of patients with sepsis, it is suggested to control the infection before starting treatment.9 History of allergic reactions should be seen as a contraindication to the parenteral formulation that caused the allergy, but reactions are generally product-dependent and it is possible to exchange for a different formulation. Intravenous iron replacement should not be performed in patients with pre-treatment ferritin levels 300–500 ng/mL and transferrin saturation >50%.10
The use of erythropoietin can be considered in patients with severe anemia, always evaluating the risks and benefits involved. Anemia of inflammation is characterized by the lack of adequate response to an increase in erythropoietin concentration due to a drop in hemoglobin. Inflammatory mediators inhibit the production of this hormone by the kidneys and limit the response in its receptors.6 Hence, anemia of inflammation is the most susceptible scenario to this therapy. Recommended erythropoietin doses vary between studies and there is no consensus protocol. If erythropoietin is used, it is recommended to administer it for a short period and in conjunction with intravenous iron. Concern about a possible increase in thrombotic risk must be considered and during the postoperative period, patients must be on concomitant use of adequate prophylactic anticoagulation.6
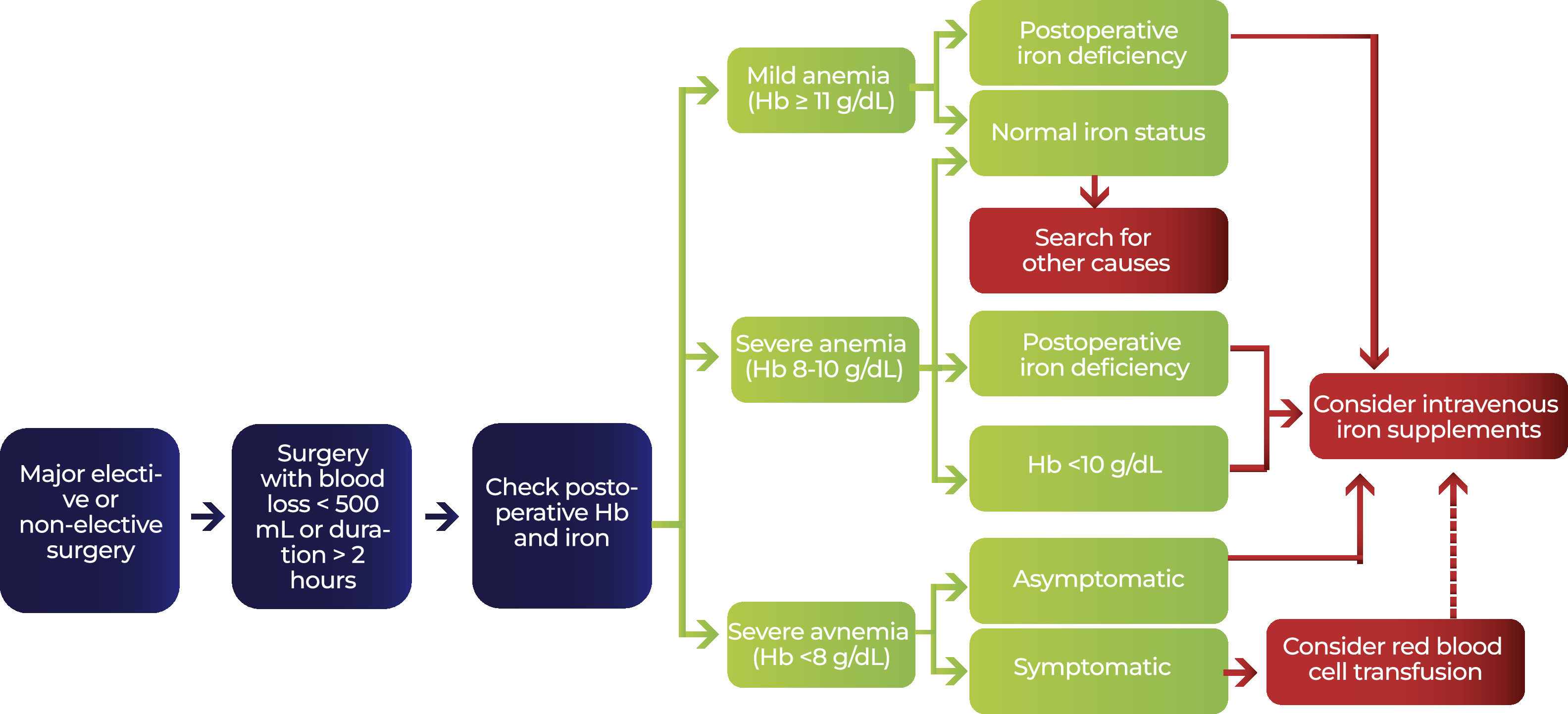
In cancer patients, the use of erythropoietin is controversial and most recommendations suggest individualizing the decision. The concern involves a possible increase in thrombotic risk in this population, in addition to a possible effect on the progression of oncological disease with a negative impact on survival.11 The current guidelines of the American Society of Clinical Oncology and the American Society of Hematology limit the use of erythropoiesis-stimulating agents in cancer patients only to those with anemia induced by chemotherapy and with a proposal for non-curative treatment when their Hb <10 g/dL.12 The European Society of Oncology recommends the use of erythropoietin in patients with cancer and other inflammatory conditions, when Hb <10 g/dL and transferrin saturation <20%, and associated with parenteral iron replacement (Fig. 1).13
Anemia associated with hospitalizationAdequate attention to the issue of postoperative anemia also involves the concept of hospitalization-associated anemia (HAA), which can be defined as anemia that develops or worsens during the period of hospitalization. 14 The prevalence of this condition varies between different studies, but is considerable, with surveys showing it affects up to 40% of patients in coronary care units and almost universal involvement in intensive care units.15
Its etiology is multifactorial, involving blood loss due to bleeding, development and progression of iron deficiency, decreased half-life of red blood cells and reduced blood production due to the effect of inflammation. However, an important component arises from iatrogenic practices, resulting in excessive phlebotomies for diagnostic exams.16 The volume of losses for collecting laboratory tests estimated in different scenarios varies from 150 to almost 500 mL, which would correspond to the volume of 1 or 2 packed red blood cells per patient per hospitalization.17
In fact, it is estimated that for every 100 mL of blood collected there is a drop of 0.7 g/dL in the patient's hemoglobin levels. 18 In some surveys, the blood volume collected was 8.5 to 12 times greater than that required for the analysis using the most modern clinical pathology equipment, leading to the discard of almost 3 mL of blood for each tube collected, which could mean, on a global scale, millions of liters of blood going ‘down the drain’ every year.19,20 In addition to being common and potentially avoidable, it is necessary to highlight that these volumes of iatrogenic losses lead to a major impact on patient outcomes, including a relationship with the subsequent need for transfusions, prolonged hospitalization and increased morbidity and mortality.21,22
Given these considerations, in addition to paying attention to anemia and iron deficiency in patients who visibly have blood loss or evident risk factors, it is necessary for centers to establish practices and protocols that minimize the occurrence and impact of anemia acquired during hospitalization. Some important attitudes may be not encouraging the collection of samples through implanted catheters, which can lower the threshold for recollections or unnecessary collections due to the easy availability of venous access. Furthermore, the use of specific pediatric tubes and point-of-care devices should be encouraged, when available, and the establishment of clinical protocols that assist the care team in greater rationalization for the request of laboratory tests.23,24
When to use transfusions of packed red blood cellsIf measures to prevent and treat postoperative anemia have not been sufficient to achieve satisfactory increases in hematimetric indices, transfusions should be indicated in a restrictive manner, reserved for patients with hemodynamic repercussions or signs of impaired tissue oxygenation, and should be assessed individually.5
Packed red blood cell transfusions should only be considered in patients with severe anemia (Hb <7 g/dL or <8 g/dL in the presence of comorbidities and clinical repercussions) or in patients with active bleeding, cases in which the clinical condition cannot be restored with volume replacement alone and the use of medications.6
Recommendations
1 - It is recommended to implement a Patient Blood Management Program in all hospitals, with the participation of specialists and a multidisciplinary team, to establish protocols, monitor their performance and promote educational actions, ensuring that anemia is adequately diagnosed and treated including in the postoperative period.
2 - Hemoglobin measurement must be carried out in all patients undergoing surgeries with expected blood loss above 500 mL or undergoing procedures lasting more than two hours, in patients with anemia diagnosed pre-operatively and in those who present moderate to severe bleeding regardless of the size of surgery. Special attention should be given to patients with risk factors for developing anemia in the postoperative period. Pay attention to patients with risk factors such as advanced age, female gender, history of anemia and excessive hemodilution during the procedure.
3 - The use of iron should be instituted in patients with evidence of iron deficiency or with a reduction in hemoglobin in the postoperative period (<11 g/dL) remembering that the diagnosis of iron deficiency during this period is more difficult since the ferritin level is elevated by surgical trauma. Preferably, intravenous iron should be used due to the greater effectiveness of parenteral replacement due to the reduced absorption of oral iron in the presence of inflammation.
4 - The use of erythropoietin can be considered in patients with severe anemia and anemia secondary to inflammation. It is recommended to use in association with intravenous iron and for a short period considering the possible risks and benefits.
5 - Blood transfusions should be reserved for patients with symptomatic anemia, with hemodynamic repercussions and using a restrictive transfusion strategy (Hb <7 g/dL or <8 g/dL in the presence of comorbidities).
6 - Pay attention to anemia associated with hospitalization and iatrogenic blood loss due to excessive phlebotomies for laboratory tests.
Effective management of postoperative anemia requires strategies tailored to the severity and origin of the condition, as well as to the patient's overall health. In more serious cases, blood transfusion may be considered, albeit with caution, due to the potential associated risks. A multidisciplinary approach is a fundamental guideline for the successful management of postoperative anemia. Involving nutritionists, hematologists and other specialties, this approach aims to address not only the clinical manifestations of anemia, but also its underlying causes. Furthermore, awareness of the potential risks of excessive phlebotomy should be promoted among healthcare professionals, encouraging a more careful assessment of the frequency and volume of blood withdrawn. Therefore, an in-depth understanding of etiological characteristics, risk factors, diagnostic methods and management approaches is essential to optimize clinical outcomes and improve the quality of care in the postoperative context.