Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoAnemia is a pathological condition in which the hemoglobin and red blood cell mass decrease; it is mainly defined by the concentration of hemoglobin in the blood. The World Health Organization guidelines establish specific values to define anemia in different population groups. Early detection of anemia can also be a valuable indicator of underlying medical conditions. Clinical studies have explored the relationship between perioperative anemia and morbidity, highlighting the need for more judicious therapeutic strategies, such as the use of Patient Blood Management, which aims to prevent and treat anemia in a personalized and effective way. Patient Blood Management emerges as a promising approach to dealing with anemia, recognizing that its correction through transfusion always carries risks and that personalized prevention and treatment can offer better outcomes for patients.
Anemia is, as a rule, a pathological condition in which there are reductions in hemoglobin (Hb) and red blood cell mass. The decrease in Hb concentration, in itself, does not define anemia, as this finding can occur in physiological situations attributed to hemodilution, such as that observed during pregnancy. Still, for practical purposes, Hb concentration (or hematocrit) is the laboratory parameter most used to define anemia. The World Health Organization (WHO) defines anemia as an Hb concentration below 12 g/dL for premenopausal women and 13.0 g/dL for men and postmenopausal women, both values considered at sea level. The parameters of normal Hb concentration in children and adolescents also differ from those observed in adults. Between 6 months and 5 years of age, the lower limit is 11.0 g/dL; between 6 and 11 years old, 11.5 g/dL; between 12 and 14 years old, 12.0 g/dL, a value that remains for women from then on. For male adolescents, the lower limit is more difficult to determine given the variability of testosterone levels at each age until the end of adolescence.1
One of the primary functions of blood circulation is to effectively supply oxygen to the cells of the different tissues. Since oxygen has limited solubility in blood plasma, an ample supply of oxygen depends on the presence of red blood cells, which have the unique ability to bind to oxygen through Hb. The sigmoidal curve of oxygen dissociation from Hb ensures the uptake of oxygen in the lungs and its release in peripheral tissues. As a consequence, oxygen exchange by red blood cells is a passive process predominantly influenced by the local microenvironment; this environment undergoes significant changes in septic patients. Normally, tissues have a venous oxygen saturation of 65 %, indicating that only about one-third of the transported oxygen is released to nourish the tissues, while at least two-thirds circulates back to the right heart through the venous circulation.2
A reduction in Hb mass can lead to a decrease in the blood oxygen transport capacity, which becomes more evident under conditions of sudden increase in demand. Reductions in Hb lead to compensatory mechanisms of the body, such as an increase in cardiac output, the redistribution of blood flow to vital organs, an increase in the influx of liquid from the extravascular to the intravascular space, among others, mechanisms that are triggered in a less effective way in the elderly or in debilitated individuals.3
Animal studies have shown the physiological changes that occur when isovolemic anemia is provoked in a controlled manner. For example, a study on mice showed that deaths began to occur only when the Hb concentration fell to levels below 5.0 g/dL and reached 100 % of the animals when Hb was below 2.0 g/dL. 3 Similar results were observed in pigs and baboons. 3 In previously healthy humans subjected to isovolemic anemia, no signs of tissue hypoxia were observed up to a Hb level of 5.0 g/dL.4 It is important to emphasize that the animals and humans subjected to induced anemia were all healthy and were kept at rest. Therefore, conclusions obtained in clinically controlled conditions cannot be extrapolated to the situation that occurs, for example, in surgical trauma, in which additional physiological demands are possibly more difficult to meet with such low Hb levels.5
According to the literature, a patients' tolerance for maintaining severe anemia, called ‘anemia tolerance’, differs depending on the situation (i.e., intraindividual differences) and the patient (i.e., interindividual differences). This is because individual tolerance to anemia depends on coexisting diseases, the individual cardiopulmonary compensation reserve and the specific situation.
In fact, opinions about what the ‘critical level’ of Hb would be differ among authors, with some postulating a critical Hb concentration of 4–5 g/dL. The common denominator underlying these very different opinions is the inconstant behavior of several ‘non-Hb variables’ that influence tissue oxygenation in addition to Hb or hematocrit. Abnormalities of these ‘non-Hb variables’ are found more frequently in critically ill patients. For this reason, the ‘critical’ Hb or hematocrit is an individual value. 6
Evidence demonstrating that a critical Hb level is an individual characteristic can be found in a literature review of case reports published to assess survival in patients with extreme anemia, defined by researchers as anemia in which Hb levels were less than 2 g/dL. A total of 23 published case reports were found to have this level of anemia. In these 23 reports, there was one death in a patient who received a blood transfusion and four patients had complications after the transfusion (posterior reversible encephalopathy syndrome). A total of four patients were not transfused because they refused to receive blood components and obtained other types of treatment, such as erythropoietin, iron, vitamin supplements, among others. The four patients survived.7
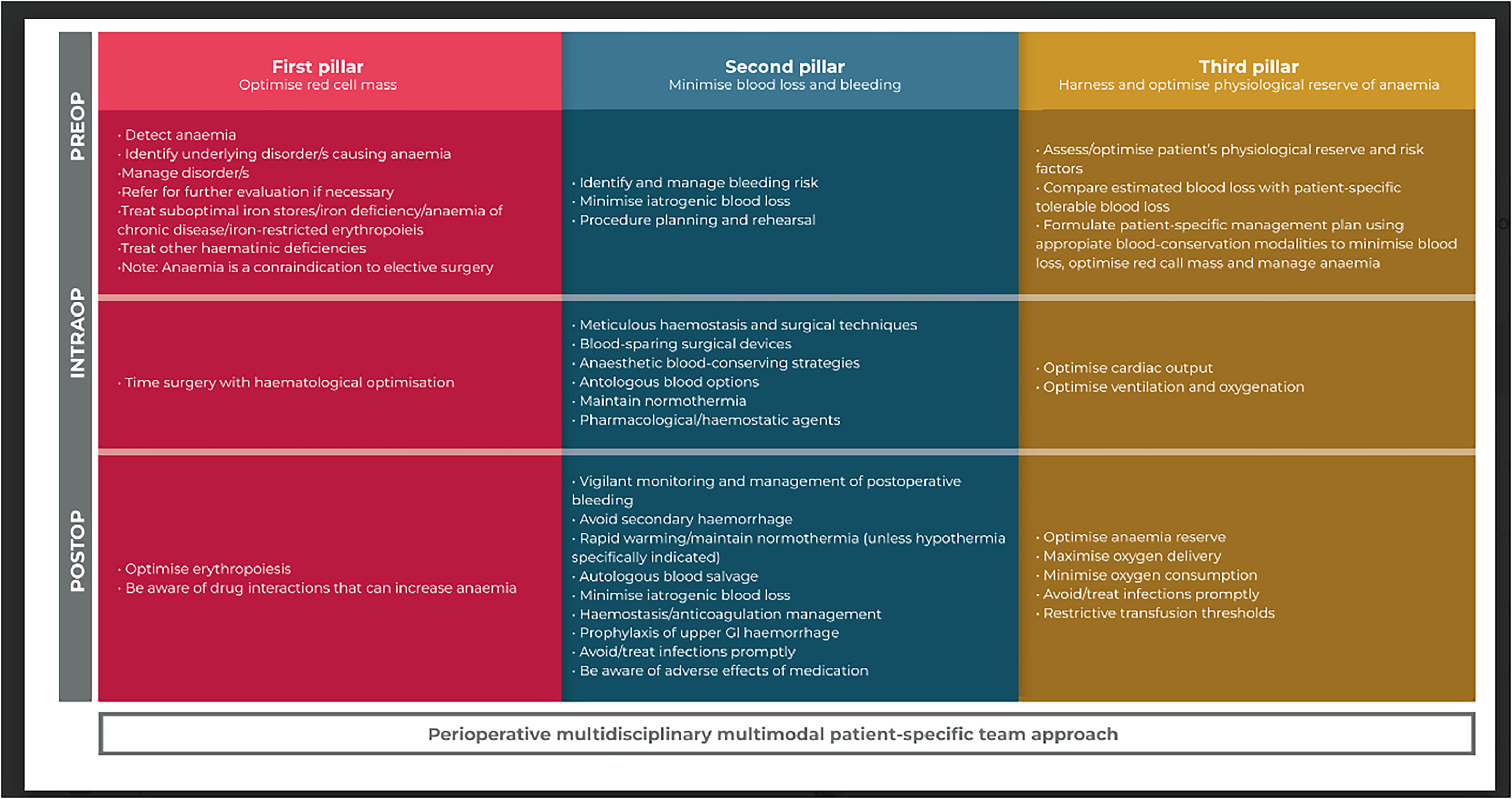
Despite the complex scenario, it is worth mentioning that the use of strategies that optimize anemia tolerance is an essential element of the third pillar of modern Patient Blood Management (PBM) programs.8
Meeting the challenge posed by certain observational studies carried out in the USA, which examined the implications of postoperative anemia in patients who had refused red blood cell transfusions based on religious beliefs, 9,10 reveals a complex relationship between Hb levels, morbidity and mortality. These studies have clarified the fact that low Hb concentrations do not always directly correlate with higher mortality rates. In recent years, there has been increasing recognition that more restrictive transfusion thresholds may be not only safe but also beneficial in specific clinical situations.11-17
Traditionally, the threshold for red blood cell transfusion to prevent morbidity and mortality has been set at a Hb concentration of about 7–8 g/dL. However, research has demonstrated that patients can tolerate lower Hb levels and that more conservative approaches to transfusion are often justified. In certain clinical contexts, such as stable patients without bleeding or with specific comorbidities, more restrictive thresholds have been adopted, as low as 6 g/dL, without compromising patient outcomes. This approach recognizes that individual patient factors and clinical conditions play a significant role in determining the appropriate transfusion threshold. Ultimately, these findings underscore the importance of a personalized, patient-centered approach to transfusion medicine, in which the focus shifts from Hb concentrations alone to a more comprehensive assessment of the patient's overall clinical status.18
The clinical point of view: the effect of acute anemia and transfusion on morbidity and mortalityIn the daily clinical practice, examining morbidity and mortality associated with acute anemia without the influence of transfusion proves to be a formidable challenge. Ethical considerations make it unfeasible to carry out a potential clinical study that includes a control group with severe anemia for a prolonged period. Consequently, most clinical investigations choose to compare groups of patients following liberal or restrictive transfusion regimens. These studies cover diverse patient populations with different underlying pathologies, making direct comparisons between them complex. Overall, these studies juxtapose the consequences of prolonged acute anemia with a possible transfusion at a lower threshold (often referred to as a restrictive transfusion regimen) against the results of transfusion with allogeneic blood after a brief period of acute anemia (liberal transfusion regimen). Although this approach is in line with common clinical practice, it is important to note that such studies cannot accurately delineate a safe lower limit of acute anemia with respect to oxygen transport. Instead, its main utility lies in offering valuable information about optimal therapeutic strategies in clinical settings.
As demonstrated by data presented in numerous studies,19-26 perioperative anemia is correlated with increased perioperative morbidity and mortality. Simultaneously, compelling evidence has highlighted that indiscriminate use of red blood cell transfusions does not improve clinical outcomes. 26 Consequently, it is imperative to take advantage of all accessible therapeutic interventions to mitigate anemia and minimize the need for allogeneic blood transfusion with the ultimate goal of improving patient outcomes. This imperative gives rise to the third paradigm, namely, the PBM perspective.18
The third pillar of PBM (Figure 1) investigates the crucial issue of determining the Hb threshold at which red blood cell transfusions become necessary in specific physiological scenarios (such as hypotension, hypoxia, sepsis, etc.) to prevent harm to the body. It is in this third pillar of PBM that opportunities to take advantage of the body's inherent tolerance to physiological anemia are defined, allowing for a lower level of Hb or oxygen transport. However, it is essential to clarify that this approach does not advocate tolerance of anemia until it reaches a predefined Hb threshold for transfusion. Instead, the overall goal is to proactively prevent any form of anemia as far as possible.18
Adapted from Thomson et al. (2019). Thomson, A Hofmann, C A Barrett, A Beeton, G R M Bellairs, L Boretti, M J Coetzee, S Farmer, M W Gibbs, H H Gombotz, C Hilton, C Kassianides, V J Louw, C Lundgren, J N Mahlangu, C B Noel, V Rambiritch, F Schneider, E Verburgh, P-L Wessels, P Wessels, R Wise, A Shander (2019). Patient blood management: A solution for South Africa. South African Medical Journal. 109. 471.27
Strategies to optimize patient tolerance to anemiaMaximize oxygen supplyArterial oxygen content (CaO2) is defined as the sum of oxygen bound to Hb and physically dissolved oxygen. During acute anemia, physically dissolved oxygen in plasma becomes a relevant component for tissue oxygenation.
It has been shown in animal models that, at the critical Hb concentration, hyperoxic ventilation increases the survival rate, increases tolerance to anemia and ensures tissue oxygenation even at very low Hb concentrations. There are also case reports of clinical success with this type of intervention.8
Maximizing oxygen supply can be done by increasing the fraction of inspired oxygen (FiO2). Another way to maximize oxygen supply is through hyperbaric oxygen therapy. A systematic review of the literature found 35 publications that evaluated the effect of oxygen therapy in the treatment of severe anemia with all studies in the review pointing to favorable outcomes. 28 Resolution 1457/95 of the Brazilian Federal Council of Medicine, which regulates oxygen therapy, includes acute anemia as a recognized clinical condition in cases where blood transfusion is impossible. It is worth noting that oxygen supplementation can lead to unwanted adverse effects, such as hyperoxic arteriolar constriction, formation of oxygen radicals, neurological toxicity and formation of atelectasis, which is why its use should be used after a risk-benefit analysis case by case.
Minimize oxygen demandMaintenance of sedation, neuromuscular paralysis and intubation with mechanical ventilation can be used to minimize the patient's oxygen consumption and demand.29
Rapid treatment of infectionsSpecial attention should be paid to infections, especially those with the potential of sepsis. From a physiological point of view, infectious conditions are accompanied by an increase in oxygen demand. Therefore, rapid and effective treatment of infections is recommended to maximize tolerance to anemia. 8 Furthermore, processes that contribute to hospital-acquired infections should be minimized, including nasogastric tubes and Foley catheters.
Greater tolerance to anemiaScientific studies have identified great variations in the indication for blood transfusion in different centers for the same clinical scenario with the same degree of anemia.30 Such data suggest that cultural and behavioral aspects, and not recommendations based on scientific evidence, should be involved in the indications for blood transfusion.
Furthermore, several scientific studies have demonstrated that patients in different clinical scenarios, including heart surgery, can tolerate moderate degrees of anemia without harm from a clinical point of view. One of these studies concluded that it is the doctor, not the patient, who does not tolerate low hematocrit levels during cardiac surgery.31 Adherence to the principles of evidence-based medicine may help doctors develop greater tolerance to anemia.
Recommendations
- 1.
More restrictive transfusion thresholds may be not only safe but also beneficial in specific clinical situations.
- 2.
Take advantage of all accessible therapeutic interventions to mitigate anemia and minimize the need for allogeneic blood transfusion, with the ultimate goal of improving patient outcomes.
- 3.
Take advantage of the body's inherent tolerance to physiological anemia, allowing a lower level of Hb or oxygen transport, acting on the causes of anemia and keeping the patient stable, within their hemodynamic and physiological thresholds.
- 4.
We recommend maximizing oxygen delivery in severely anemic patients who show signs of tissue hypoxia after a case-by-case risk-benefit analysis.
- 5.
We recommend strategies to minimize oxygen demand.
- 6.
We recommend rapid treatment of infections.
The notion of anemia tolerance has received substantial attention in recent literature, but it encompasses a spectrum of diverse clinical scenarios. Mainly, it means the body's ability to withstand acute and normovolemic anemia. However, it also encompasses the recognition that both mild and moderate anemia serve as independent, long-term risk factors for morbidity and mortality, emphasizing the need for their prevention or appropriate management. Correction of mild to moderate anemia through red blood cell transfusions rarely leads to better results in the general population and should be reserved for specific indications, such as acute hemorrhages with hemodynamic repercussions. As a promising alternative, Patient Blood Management (PBM) offers a robust toolkit for the prevention and treatment of anemia.
Ethical considerations in the conduct and reporting of research: protection of human subjects and animals in researchWhen reporting experiments on human subjects, authors should indicate whether the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. If doubt exists whether the research was conducted in accordance with the Helsinki Declaration, the authors must explain the rationale for their approach and demonstrate that the institutional review body explicitly approved the doubtful aspects of the study. When reporting experiments on animals, authors should indicate whether the institutional and national guidelines for the care and use of laboratory animals were followed.