Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoUnderstanding the physiological concepts of oxygen delivery is essential to discern the mechanisms that influence its increase, reduction or maintenance in the body. This text explores the different mechanisms that help maintain oxygen delivery even in the face of reduced hemoglobin levels. Adequate oxygen delivery ensures tissue and metabolic balance, which is crucial to avoid harmful consequences such as metabolic acidosis and cellular dysoxia. The complex interaction between variables such as cardiac output, hemoglobin and heart rate (HR) plays a fundamental role in maintaining oxygen delivery, allowing the body to temporarily adjust to situations of anemia or high metabolic demand. It is important to emphasize that blood transfusions should not be based on fixed values, but rather on individual metabolic needs. Strategies to reduce myocardial consumption and monitor macro and micro hemodynamics help in making rational decisions. Individualizing treatment and considering factors such as blood viscosity in relation to the benefits of transfusion are increasingly relevant to optimize therapy and minimize risks, especially in complex clinical scenarios, such as neurocritical patients and trauma victims.
Understanding the physiological concepts of oxygen delivery (DO2) is fundamental to understanding the variables that are capable of increasing, reducing or maintaining the supply of oxygen (O2) to the body. Based on this reasoning, this text discusses the possible mechanisms for maintaining DO2 even with reduced hemoglobin levels.
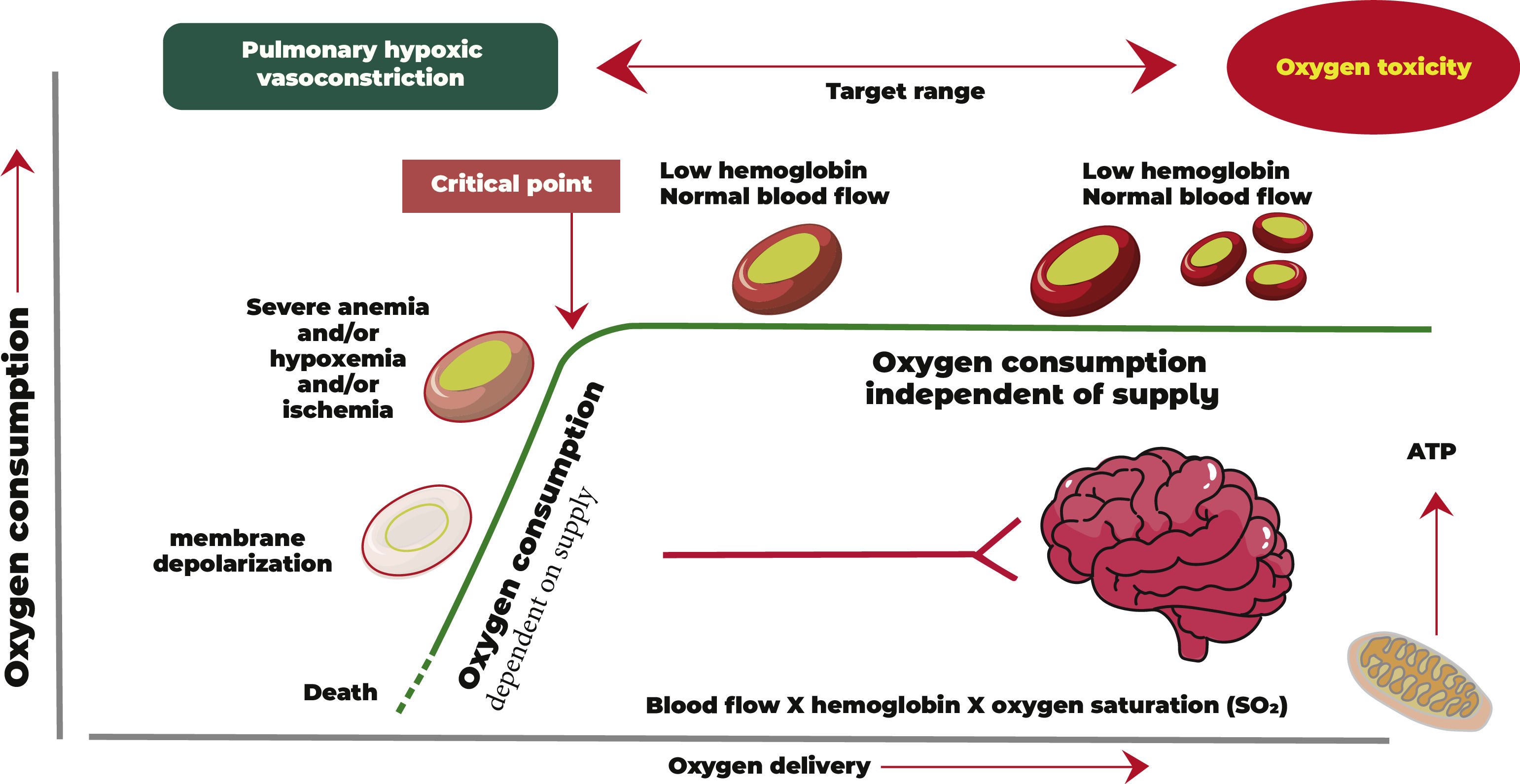
Adequate O2 supply guarantees tissue homeostasis within a physiological pattern of dynamic balance (Figure 1). This balance is guaranteed through adequate DO2 and nutrients and the removal of carbon dioxide, free radicals and other metabolic products. With a multifactorial cause, the imbalance between supply and demand can lead to harmful consequences for the body, such as accumulation of acidic metabolic products and, consequently, metabolic acidosis, cellular dysoxia, multiple organic dysfunctions, prolonged hospitalization and even death.1,2
As can be seen from Figure 1, O2 distribution is a product of blood flow and arterial O2 content. When the DO2 decreases below a critical point, O2 consumption is impaired, leading to anaerobic metabolism and lactic acidosis.
It is well known that prolonged cellular dysoxia, both in the surgical and intensive care environments, is associated with increased mortality. One of the vital goals of healthcare professionals is ensuring the adequacy of DO2 in critically ill patients, whether in the operating room or in the intensive care unit (ICU).
To better understand the mechanisms of anemia tolerance, it is important to remember the DO2 variables and their physiological interactions.
Physiological aspects of oxygen deliveryDO2 is a consequent product of arterial O2 content and cardiac output (CO). Therefore, it is necessary to understand all related variables with their deficits and treatment potential to optimize DO2. DO2 is calculated by Formula 1.
Formula 1 Oxygen delivery
DO2 = oxygen delivery; CaO2 = arterial oxygen content; CO = cardiac output
The arterial oxygen content (CaO2 - Formula 2 below) is guaranteed, for the most part, by the amount of O2 transported bound to hemoglobin and, to a lesser extent, by the amount of O2 transported dissolved in the plasma. It is known that the solubility coefficient of O2 in plasma is low and, therefore, a small quantity of O2 is transported. Therefore, increases in DO2 are not possible through this means of transport under normal temperature and pressure conditions. On the other hand, we are aware that a hemoglobin molecule carries up to 1.37 mL of O2, that is, the DO2 can be increased by improving the hemoglobin level. However, it is known that very high hemoglobin levels are associated with worse viscosity and thus worse blood flow and that poorly indicated transfusions, for this reason alone, can worsen patients' clinical outcomes. It is also important to highlight that as the carrying capacity of hemoglobin is also finite (1.37 mL x Hb), this means that we cannot achieve a “scaled and proportional” improvement in DO2 through an isolated increase in the fraction of inspired O2 (FiO2).
Formula 2 Arterial oxygen content
CaO2 = arterial oxygen content; Hb = hemoglobin in grams per 100 mL of blood (14 to 15 g/dL); SaO2 =% oxyhemoglobin - fractional saturation of hemoglobin; 1.37 = number of milliliters of O2 linked to 1 g of saturated Hb; 0.003 = O2 solubility in plasma, vol% mmHg refers to the arterial blood sample; PaO2: Partial pressure of oxygen
Hypoxemia is defined as a reduction in arterial O2 content, while cellular dysoxia is defined as a metabolic imbalance, either due to a lack of O2 or due to an inadequate flow, which creates an imbalance between the generation and removal of toxic radicals resulting from cellular metabolism. Therefore, reductions in DO2 can lead to tissue dysoxia. An imbalance between delivery and demand leads to increased production or accumulation of markers resulting from anaerobic metabolism such as lactate, which can be measured in the laboratory.
Another important point to understand DO2 is CO (Formula 3), where CO is the product of stroke volume and HR.
Formula 3 Cardiac output
DC= cardiac output; SV= stroke volume; HR= heart rate
When analyzing CO variables, we know that the systolic volume will have its performance subordinated to the physiological properties of the myocardium such as preload, afterload and contractility, that is, changes in volume, in systemic vascular resistance and in contractility can lead to changes (for more or for less) in the CO and, consequently, impact DO2. Patients with very high systemic vascular resistance may experience worsening of myocardial function due to increased cardiac work and a consequent drop in DO2. On the other hand, dramatic drops in systemic vascular resistance can also generate tissue perfusion pressures so low that blood flow becomes unsatisfactory to meet the patient's tissue metabolic demand.3
Patients with impaired myocardial performance, such as those with severe heart failure or in cardiogenic shock, may not have sufficient stroke volume to guarantee tissue demand. This situation can also generate a drop in DO2, an increase in lactate, an accumulation of metabolic waste (metabolic acidosis and renal failure) and a ‘slower’ venous return which can be observed in the laboratory with an increase in delta CO2 (difference in CO2 between arterial and venous blood gases of >6 mmHg) and a drop in central venous saturation (less than 65–70%).
It is always very important, however, to individualize the conduct for each patient, closely observing their responses to interventions and their evolution. It is not true that every heart patient needs high hemoglobin values to improve their DO2. In a systematic review that included patients submitted to cardiac surgery, it was concluded that a restrictive transfusion strategy of 7–8 g/dL is safe and reduced the use of red blood cell transfusions by 24%. The review also emphasizes that more research is necessary to define the ideal transfusion threshold in patients with acute myocardial infarction.4
Changes in HR tend to impact DO2 at its extremes. Very high frequencies that are not compatible with age (e.g., tachyarrhythmias) can impair systolic filling and coronary flow and, consequently, reduce CO and DO2. However, extremely low HRs (symptomatic bradycardias) can also impair CO and there is also a drop in coronary flow.3,5
Our organic systems interact to seek homeostasis and balance so that, even in cases of extreme anemia or a drop in stroke volume (e.g. hemorrhagic shock, extensive acute myocardial infarction), CO will try to compensate and maintain DO2 typically by increasing the HR. On the other hand, a significant reduction in HR or exaggerated pathological increases (tachyarrhythmias) will generate a state of tissue hypoperfusion and activation of sympathomimetic receptors. This activation of receptors leads to a reflex increase in systemic vascular resistance (SVR), in the body's compensatory attempt to maintain tissue perfusion pressure and guarantee DO2, and coronary and cerebral perfusion. It is known, however, that this persistent increase in SVR can result in impaired organic perfusion and lead to cellular dysoxia if the cause of the imbalance is not resolved.
It is extremely important to remember that all of these compensatory mechanisms have synergistic and temporary effects and that if the underlying cause of the ‘disorder’ is not resolved, the patient may progress to refractory shock and death.
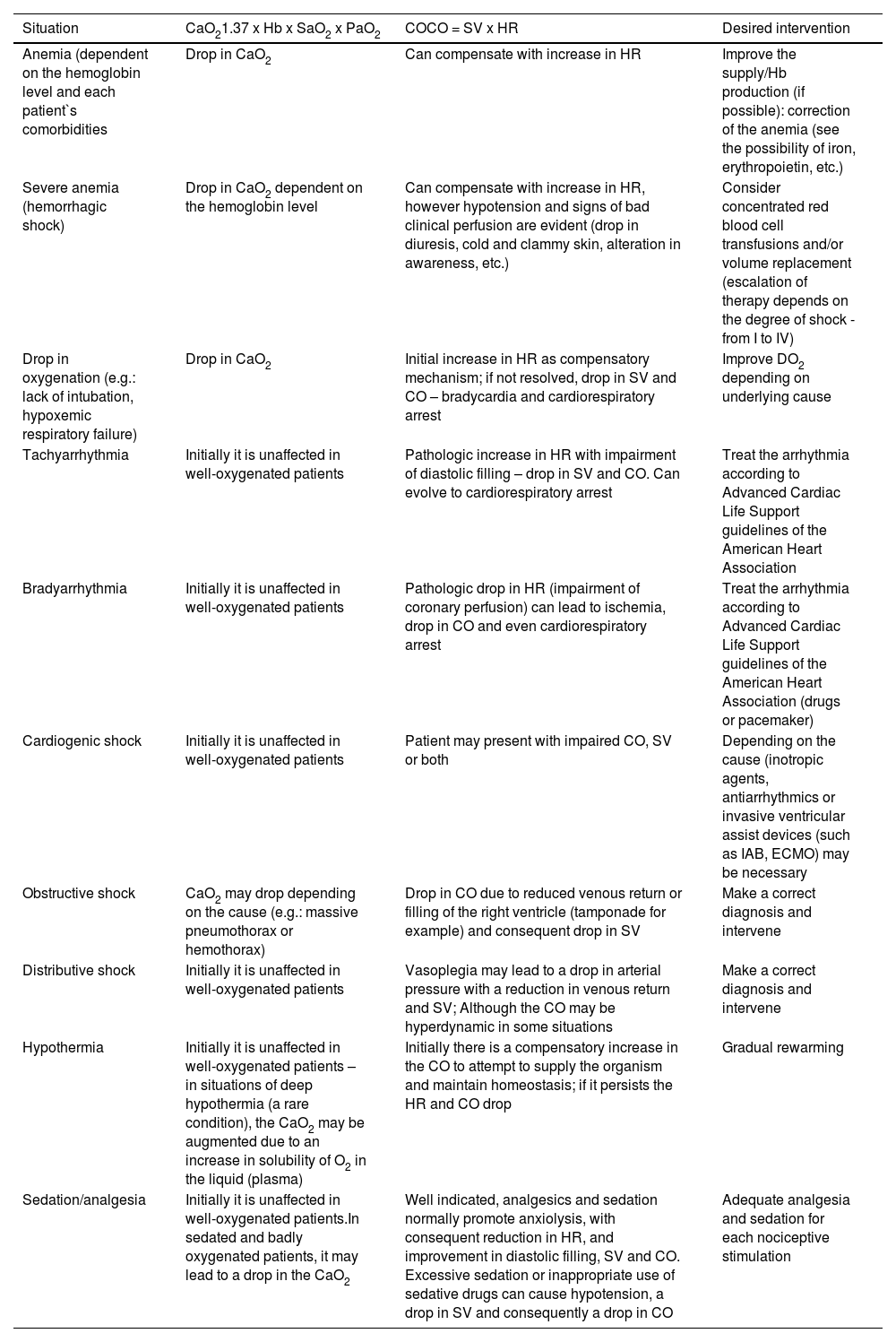
Table 1 illustrates the main clinical situations related to the variables affected by poor DO2 and the necessary interventions.
Correlation between the main clinical situations and the variables affected by oxygen delivery.
CaO2: Arterial oxygen content; Hb: Hemoglobin; SaO2: Oxygen saturation; PaO2: Partial pressure of oxygen; CO: Cardiac output; SV: Stroke volume; HR: Heart rate; IAB: intra-aortic balloon; ECMO: extracorporeal membrane oxygenation.
Laboratory markers and diagnostic devices can be used to monitor tissue perfusion. However, in the clinical practice, many of these devices are expensive and are not readily available in healthcare units. In most services, there is arterial and venous blood gas analysis for decision making. With blood gases, indirect signs of anaerobic metabolism are observed as likely reasons for the drop in DO2. It is very important to correlate clinical data with laboratory findings always. Therefore, the presence of metabolic acidosis may be a frequent finding in initial cases of shock. The progressive drop in bicarbonate, as well as more negative base excess (BE) values, should make the healthcare professional pay attention to a likely progressive to hypoperfusion even if the blood pressure is maintained.
Lactate is the result of anaerobic metabolism and its progressive increase should serve as a warning about a metabolic deviation that is taking place; measures must be initiated to begin its ‘clearance’. Its rise to critical levels is associated with increased mortality especially in septic patients and trauma victims. Delta CO2 is the difference in CO2 between venous and arterial blood gases. When this difference is wide (>6 mmHg), this indicates tissue low blood flow.
The reduced blood flow through tissues (e.g. cardiogenic shock) in cases of anemia can also lead to a drop in central venous saturation (SvO2). Therefore, it can also be an indirect indicator of tissue hypoperfusion.
Although several factors affect the SvO2 causing overestimations even with a concomitant drop in DO2 (e.g.: hypothermia, sepsis, hyperdynamic patients), low values tend to be more valued when analyzed within the patient's clinical context.
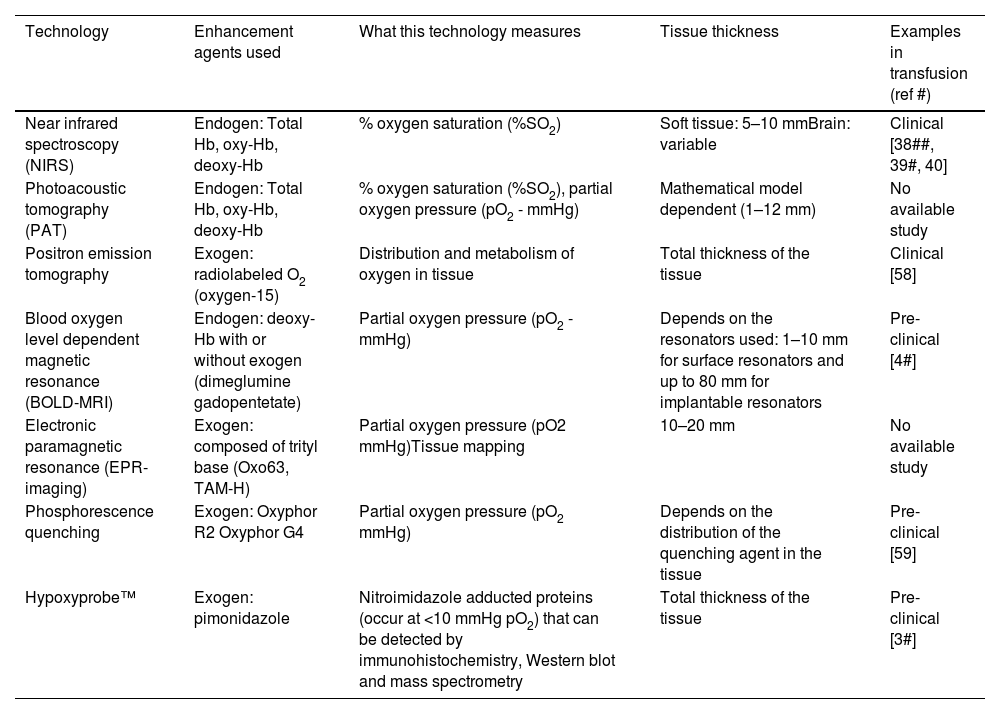
Non-invasive or minimally invasive technologies can provide relevant information about tissue O2 before and after red blood cell transfusions. The application of oximetry techniques, such as electronic paramagnetic resonance (EPR-imaging), near infrared spectroscopy (NIRS), photoacoustic tomography (PAT) and blood oxygen level dependent magnetic resonance (BOLD-MRI), are interesting opportunities to measure O2 concentration (EPR) and saturation (NIRS, PAT and BOLD-MRI - Table 2).6 Unfortunately, due to their high cost, they are not yet widely available.
Technologies that measure tissue oxygen with potential use for transfusions.
oxy-Hb: oxyhemoglobin; deoxy-Hb: Deoxygenated hemoglobin.
Adapted from Bock and Buchler (2019).
In view of what was previously discussed, it is more understandable that the mechanisms of tolerance to anemia depend directly on DO2 variables. Thus, strategies that reduce myocardial consumption, which range from analgesia to HR control, are applicable in different scenarios in the clinical practice.
Another important point that we must remember is that patients should not be transfused considering fixed parameters, but rather based on metabolic needs.7,8 Monitoring macro hemodynamics and, more notably, micro hemodynamics is extremely important in making a rational decision. A clear example that we can highlight is neurocritical patients, victims of trauma, in which the ideal hemoglobin is not yet a well-established consensus. Although responsible for delivering O2, it is also known that increases in hemoglobin can lead to increased viscosity with worse regional microcirculatory in some clinical situations.9 Given this, increasingly new ways to monitor and individualize patients are being considering in an attempt to meet their real needs and rationalize transfusion indications.
Recommendations
- 1.
Directly or indirectly monitor whether oxygen delivery is adequate in critical patients.
- 2.
Measures to improve metabolism should be a priority in patients with signs of hypoperfusion or clear signs of poor tissue perfusion.
- 3.
Transfusion of packed red blood cells should not be based on pre-established numbers; it is recommended that decisions be individualized for each patient, taking into account their comorbidities, their current clinical status and tissue perfusion markers.
The main objective of the clinical indication for red blood cell transfusion is to restore or maintain adequate oxygenation. Oxygen transport after transfusion is affected by numerous factors such as perfusion, allosteric saturation/desaturation of hemoglobin and tissue oxygen concentration.
Bioavailable oxygen maintains tissue homeostasis. Homeostasis imbalance can be measured indirectly by tissue and technological markers at the bedside. The ‘active search’ for this imbalance in critically ill patients can be valuable to assess the quality of oxygen delivery, the real need for transfusions and, thus, guide appropriate action.