Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoManaging anemia before surgery is extremely important as it is a clinical condition that can significantly increase surgical risk and affect patient outcomes. Anemia is characterized by a reduction in the number of red blood cells or hemoglobin levels leading to a lower oxygen-carrying capacity of the blood. Proper treatment requires a multifaceted approach to ensure patients are in the best possible condition for surgery and to minimize potential complications. The challenge is recognizing anemia early and implementing a timely intervention to correct it. Anemic patients are more susceptible to surgical complications such as increased infection rates, slower wound healing and increased risk of cardiovascular events during and after surgery. Additionally, anemia can exacerbate existing medical conditions, causing greater strain on organs and organ systems. To correct anemia and optimize patient outcomes, several essential measures must be taken with the most common being identifying and correcting iron deficiency.
About one third of the world's population has some degree of anemia. The World Health Organization (WHO) defines anemia as Hb <12 g/dL in women and Hb <13 g/dL in men.1 Iron deficiency is the most common cause of anemia. It can be provoked by increased physiological demand for iron (growth spurts in children, pregnancy in women), low intake (malnutrition, vegetarian or vegan diets), malabsorption (surgical causes, inflammatory disease, celiac disease) and chronic losses (menorrhagia, gastric ulcer, hematuria).2,3
The second most common cause of anemia is known as anemia of chronic disease (anemia of inflammation) and is associated with conditions such as neoplasms, chronic diseases (heart failure, kidney failure, chronic obstructive pulmonary disease) and autoimmune diseases. In anemia of chronic disease, there may be a component of functional iron deficiency, in which iron is not available for erythropoiesis. This occurs mainly due to an increase in the hormone hepcidin, which is stimulated by inflammatory cytokines.1 A small portion of anemia cases are associated with deficiencies in elements such as vitamin B12 and folic acid, due to lack of intake or conditions of malabsorption.4
The prevalence of anemia in the preoperative period is around 36 %; this varies according to demographic factors and the underlying disease. Postoperatively, the prevalence of anemia can reach 80–90 %.5 As in the general population, iron deficiency (ID) is also the most common cause of anemia among patients submitted to surgery, accounting for two thirds of patients.1 In most cases, the diagnosis of the cause of anemia can be carried out by a non-specialist doctor using laboratory tests that are widely accessible and easy to interpret.1 After initial assessment, if the cause of the anemia is not obvious, if there is another associated cytopenia (not explained by the underlying disease) or if there is evidence of other mechanisms of anemia, such as the presence of hemolysis, the patient should be referred to a specialist.
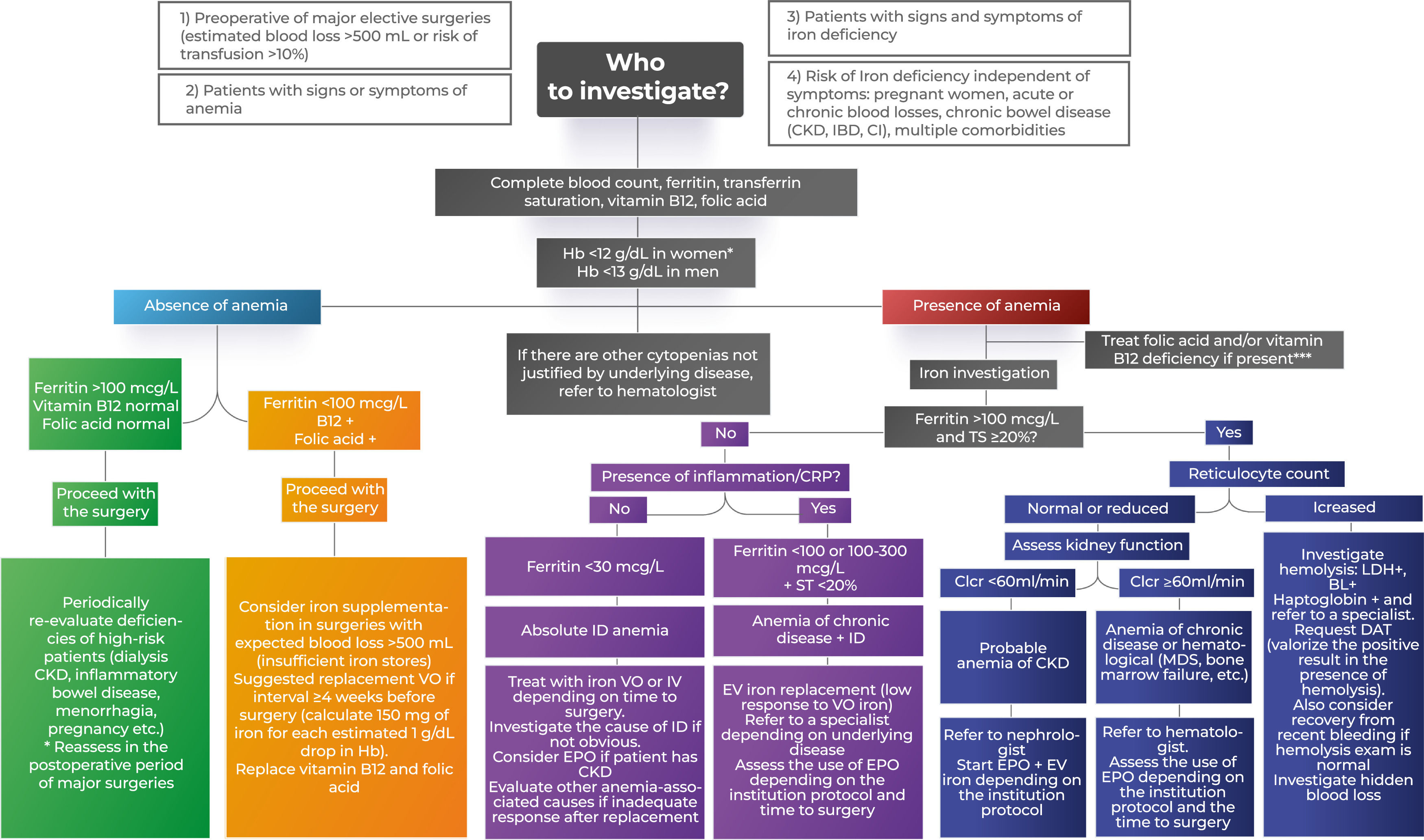
Each center must have an algorithm, adapted to its own reality, for the initial investigation of anemia in the preoperative period taking into account the complexity of the patient and the availability of tests. Every protocol must at least include a blood count and an investigation of iron levels (ferritin and transferrin saturation) for patients who will undergo surgery with a risk of transfusion >10 % or an estimated blood loss of >500 mL. Patients with signs or symptoms of anemia, ID or risk factors for both should also be investigated.1
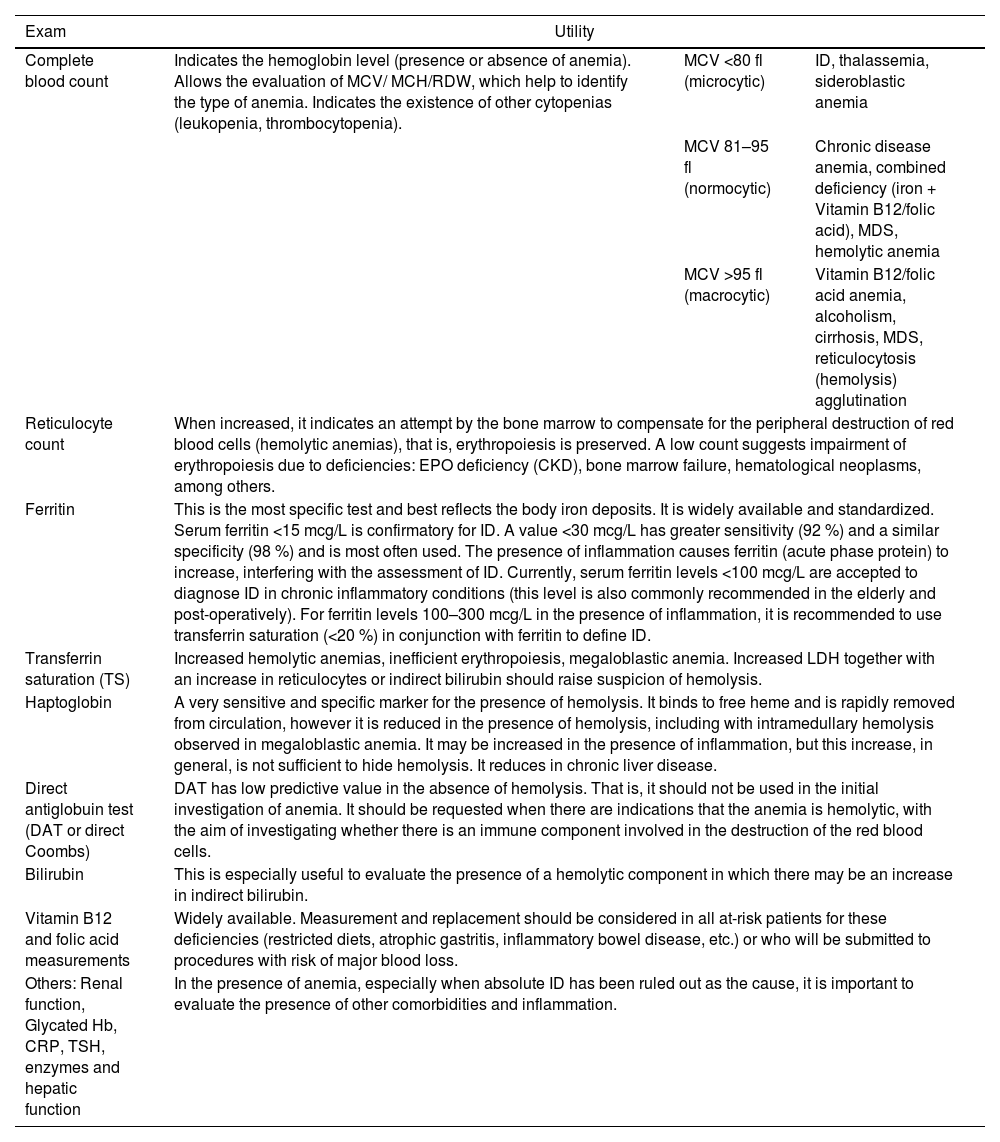
Table 1 provides a summary of the usefulness of each test in the investigation of anemia, while Figure 1 is an example of an algorithm for the preoperative approach to anemia by a non-hematologist.
Usefulness of exams in the initial investigation of anemia.
MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; RDW: Red cell distribution width; MDS: Myelodysplastic syndrome; EPO: Erythropoietin; CKD: Chronic kidney disease; ID: Iron deficiency; LDH: Lactate dehydrogenase; Hb: Hemoglobin; CRP: C-reactive protein; TSH: Thyroid stimulating hormone.
Algorithm to investigate preoperative anemia treatment.
CKD: chronic kidney disease; IBD: inflammatory bowel disease; CI: Cardiac insufficiency; VO: Via oral; EV: Endovenous; BL: Bilirubin; LDH: Lactate dehydrogenase; TSH: Thyroid-stimulating hormone; ID: Iron deficiency; EPO: erythropoietin; MDS: Myelodysplastic syndrome; CrCl: Creatinine Clearance; CRP: C-Reactive protein; DAT: Direct antiglobulin Test; TS: Transferrin saturation.
With confirmation of iron deficiency as the cause of anemia, two measures need to be implemented in parallel:
- a)
Identification of possible bleeding and its cause, if it is not evident and
- b)
The choice of the replacement therapy most appropriate for each patient and specific context, which can be carried out, in general, using one of the following three approaches:
- •
oral iron formulations.
- •
low-dose injectable iron formulations.
- •
high-dose injectable iron formulations.
- •
To define the best replacement strategy in each specific scenario, it is important to consider factors such as:
- •
Intensity of anemia and possible organic repercussions: Patients with more severe anemia, significant symptoms or with important comorbidities, such as heart disease or ischemic conditions, may not benefit completely from the use of low-dose oral or parenteral formulations due to the longer time necessary to recover erythropoiesis.
- •
Interval until the surgical procedure: Preoperatively, intravenous replacement quickens hemoglobin recovery and should be preferred over oral replacement, especially if the interval before surgery is less than six weeks.6 However, even with high-dose intravenous iron formulations, an interval of at least 10 days between the infusion and the surgical procedure is recommended to achieve a satisfactory response.7
- •
Gastrointestinal intolerance: In addition to limitations in absorption rates (maximum 25–30 mg of elemental iron/day), oral formulations can lead to considerable side effects, such as epigastric pain, heartburn, nausea and intestinal constipation, which can prevent the continuation of treatment in a significant portion of patients. Patients with active inflammatory bowel diseases may also have worsened symptoms.8
- •
Inadequate absorption: For patients with absorption problems (atrophic gastritis, gastrectomy, post-bariatric surgery, etc.) or patients with chronic or inflammatory diseases (renal failure, heart failure, inflammatory bowel disease, etc.) prefer intravenous replacement as in these cases the absorption of oral iron will be low.
- •
Availability/access: Patients in a more restricted socioeconomic context may have significant barriers to accessing injectable (especially high-dose) formulations considering the higher cost and the need for an infusion center.
- •
Venous access: Patients in need of intravenous replacement and with difficult venous access should benefit from the use of high-dose iron, as, in general, one infusion is sufficient for complete replacement.
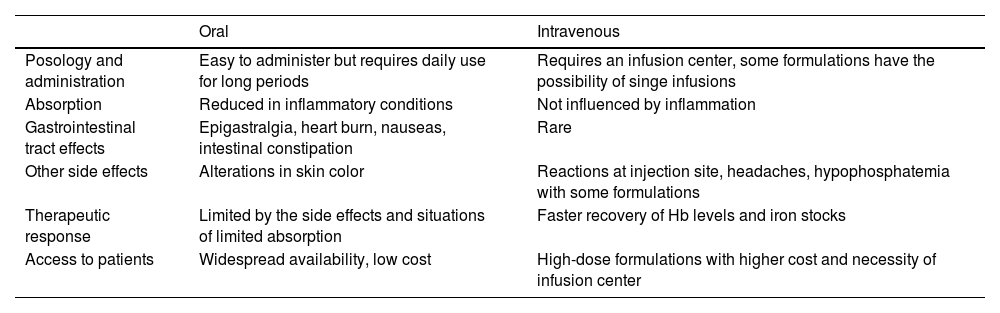
Table 2 summarizes the main advantages and disadvantages of alternatives for iron replacement.
Main characteristics comparing oral and parenteral iron formulations.
Adapted from Cappellini, Musallam KM, Taher (2020).1
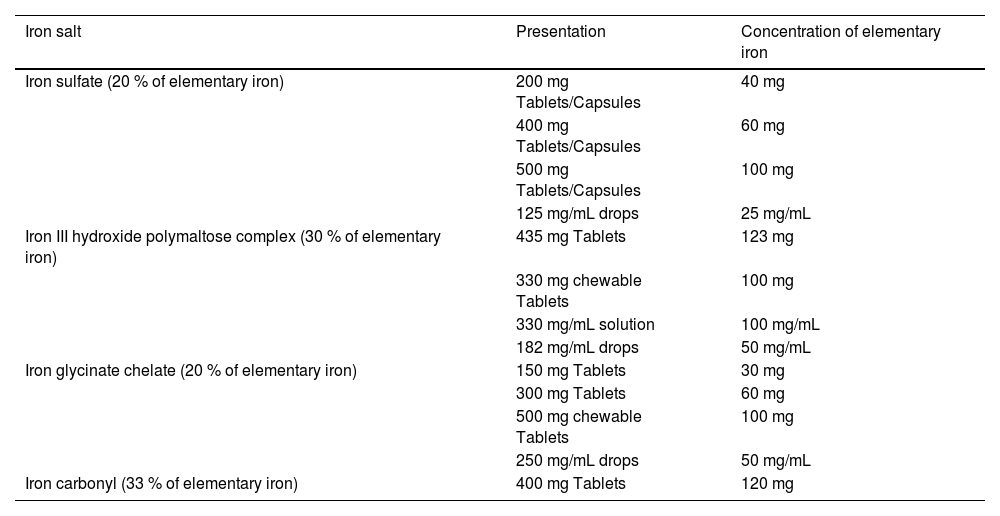
The dose traditionally recommended for iron replacement is 100–200 mg of elemental iron per day, divided into two to three doses. However, more recent studies have shown that the use of a single daily dose every other day improved the absorbed fraction of iron and reduced adverse effects.8 Thus, the current trend is to indicate smaller doses of elemental iron (60–120 mg) in a single daily intake on alternate days.9 An option also, in case of oral intolerance, is to change the oral formulation, for example, from ferrous sulfate to polymaltose iron. Table 3 shows the elemental iron concentration of some of the oral formulations.
Principal oral iron salts.
Adapted from Zago MA, Falcão RP & Pasquini; Tratado de Hematologia, 2013 Atheneu.
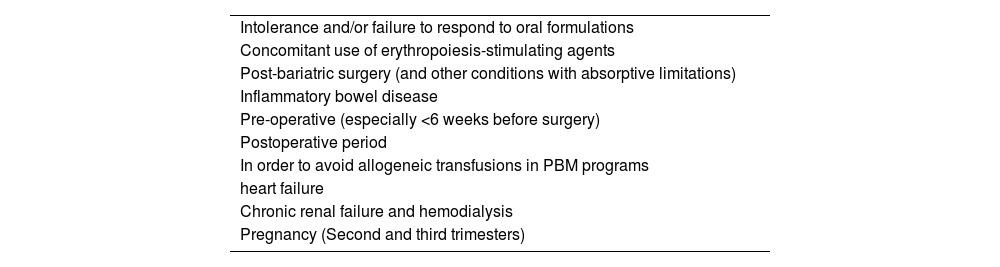
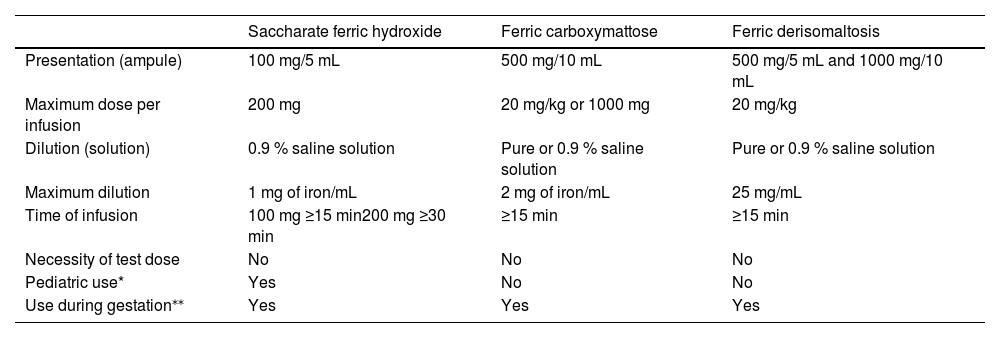
Table 4 summarizes the main indications for the use of intravenous iron, while Table 5 describes the characteristics of intravenous iron formulations available in the country. It is important to remember that although serious reactions, such as anaphylactoid reactions, are rare (<1:200,000), the infusion must be carried out in a place with trained staff and adequate structure to deal with possible complications. It is recommended that the patient remains under observation for 30 min after the end of the infusion.10
Potential indications for parenteral iron replacement.
Adapted from Cappellini, Musallam KM, Taher (2020) 1
Characteristics of endovenous iron formulations.
| Saccharate ferric hydroxide | Ferric carboxymattose | Ferric derisomaltosis | |
|---|---|---|---|
| Presentation (ampule) | 100 mg/5 mL | 500 mg/10 mL | 500 mg/5 mL and 1000 mg/10 mL |
| Maximum dose per infusion | 200 mg | 20 mg/kg or 1000 mg | 20 mg/kg |
| Dilution (solution) | 0.9 % saline solution | Pure or 0.9 % saline solution | Pure or 0.9 % saline solution |
| Maximum dilution | 1 mg of iron/mL | 2 mg of iron/mL | 25 mg/mL |
| Time of infusion | 100 mg ≥15 min200 mg ≥30 min | ≥15 min | ≥15 min |
| Necessity of test dose | No | No | No |
| Pediatric use* | Yes | No | No |
| Use during gestation⁎⁎ | Yes | Yes | Yes |
Adapted from Auerbach M., Adamson JW.; How we diagnose and treat iron deficiency anemia; AJH; 2016 and Girelli D et al.; Modern iron replacement therapy; Clinical and pathophysiological insights; International Journal of Hematology 2018;107:16–30.
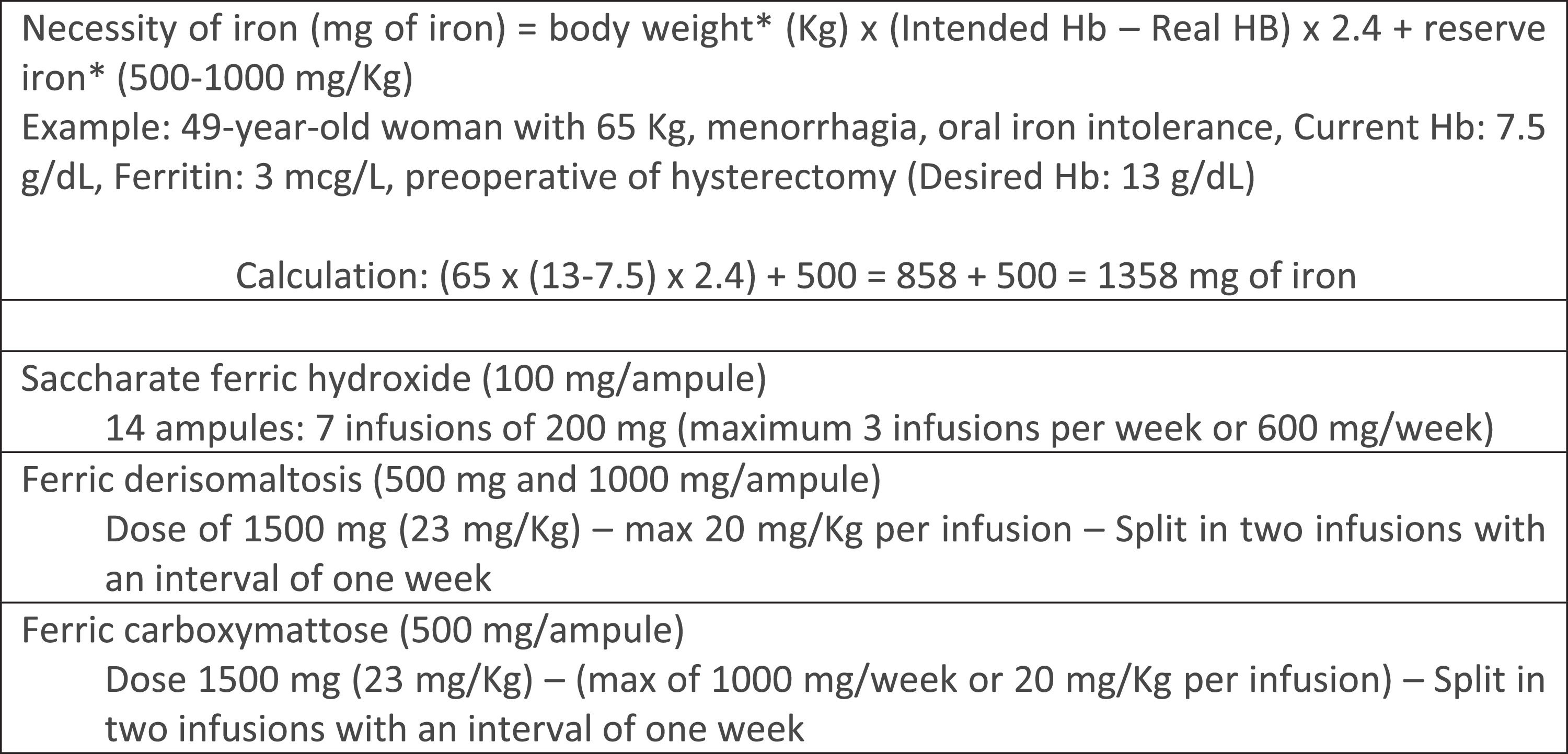
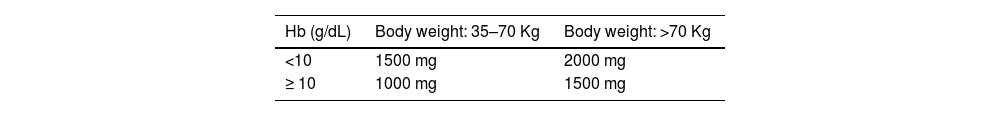
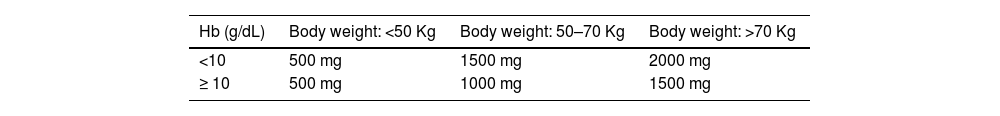
The total replacement dose of iron can be calculated using the Ganzoni formula. Figure 2 shows an example of the calculation. For high dose formulations there are also simplified tables to determine the amount to be replaced (see Tables 6 and 7).
The use of recombinant erythropoietin (rEPO) is being recommended in the presence of preoperative anemia after excluding the possibility of nutritional deficiencies, hematological malignancies and autoimmune diseases.6 rEPO can be used in preoperative treatment protocols for anemia of chronic disease, if there are no contraindications and always with parallel treatment of the underlying disease.5 Elderly patients with chronic kidney disease or myelodysplastic syndrome usually respond well to EPO. It is important to ensure that iron stores are adequate when starting EPO treatment. In this case, the use of intravenous iron should be preferred.5 rEPO has been increasingly used in Patient Blood Management (PBM) protocols to preoperatively optimize erythrocyte mass and thus reduce the number of transfusions required. Experience, especially in major orthopedic surgeries, such as hip and knee arthroplasty, shows a significant improvement in hemoglobin and a reduction in the need for transfusions, without any increase in mortality or adverse events.11 Good results are also being obtained in cardiac surgery, even with a short surgery interval, using a high dose of EPO in a small number of applications.12
The risks and benefits of EPO must always be contemplated and to do so, the underlying cause and severity of the anemia, individual characteristics of the patient, type of procedure and use of perioperative venous thromboprophylaxis must be considered. The biggest concern is the increased risk of thrombotic events, which has been observed with prolonged use of EPO targeting high hemoglobin levels (>13 g/dL) in patients with chronic kidney disease and oncological diseases.13,14 Therefore, when defining an institutional PBM protocol, the use of rEPO should not be adopted as a universal measure to correct anemia in any patient or for any procedure; it is necessary to adopt evidence-based guidelines and also individualize the patient's risk.
Recommendations
We recommend that for the effective management of patients' anemia, the following actions be observed:
- 1.
Early detection: Regular screening for anemia, especially in high-risk patients or those scheduled for elective surgery, is vital. This allows healthcare professionals to identify anemia at an early stage and implement appropriate interventions promptly.
- 2.
Identifying underlying causes: It is essential to identify and address the underlying causes of anemia, which can range from nutritional deficiencies (e.g., iron, vitamin B12 and folate) to chronic illnesses and bleeding disorders.
- 3.
Nutritional support: in cases of nutritional deficiencies, appropriate supplementation and dietary modifications should be prescribed to restore adequate levels of iron, vitamins or minerals.
- 4.
Iron replacement: Iron deficiency anemia is one of the most common types of anemia. Oral or intravenous iron supplementation may be prescribed to replenish iron stores and increase hemoglobin levels.
- 5.
Erythropoietin (EPO) therapy: In certain situations, particularly for patients unable to receive blood transfusions, erythropoietin-stimulating agents may be used to stimulate red blood cell production.
- 6.
Preoperative optimization: If surgery is planned, sufficient time should be allowed for correction of anemia before the procedure. This may involve postponing elective surgeries, when possible, to give the patient enough time to respond to treatment.
- 7.
Collaborative care: Effective management of anemia requires collaboration between different medical specialties, including surgeons, hematologists, and anesthesiologists. Each plays a crucial role in assessing and addressing a patient's anemic status and coordinating appropriate care.
By effectively managing anemia, especially before and after surgery, healthcare professionals can significantly improve patient outcomes, reduce surgical risks, and improve postoperative recovery. Early detection, addressing underlying causes and implementing necessary interventions are essential steps to ensure patients are in the best possible condition for surgery and can undergo the procedure with minimal complications.