The tumor lysis syndrome is a medical emergency. Its presentation can be spontaneous or secondary, as a consequence of the established treatment. The diagnosis and treatment of the tumor lysis syndrome (TLS) has become a crucial goal to the evolution and improvement of treatments and targeted therapies.
MethodsBetween January 2014 and December 2019, we retrospectively reviewed inpatients aged over 18 years with hematological neoplasms from a tertiary hospital in Brazil. We identified 112 episodes of TLS in 97 patients. The incidence was 10.5% and the median OS was 13.0 months (95%CI 6.2 - 19.7). The median age was 56 years (IQR 39.5 - 64). The most frequent diagnoses were multiple myeloma (18.6%), acute myeloid leukemia (17.5%) and diffuse large B-cell lymphoma (17.5%). All patients received intravenous (IV) hydration. The management also included the administration of allopurinol in 76% of the cases and rasburicase in eight patients. Renal replacement therapy was necessary in 37% of the cases. In the multivariate analysis, age, HIV status and ICU treatment were significantly associated with OS.
ConclusionThe TLS is a serious complication in the setting of hematological malignancies. The use of scores for risk stratification, as well as the inclusion of prophylactic measures and prompt treatment with frequent laboratory monitoring, is essential to reduce morbidity and mortality in the comprehensive treatment of these patients.
The tumor lysis syndrome (TLS) is a medical emergency in the hemato-oncological patient. Its presentation can be spontaneous or secondary, as a consequence of the established treatment. Laboratory abnormalities are developed by the rupture of homeostasis due to the rapid lysis of tumor cells that trigger the release of potassium, phosphate and uric acid, resulting in hyperkalemia and hyperphosphatemia, with secondary hypocalcemia and hyperuricemia.1,2
The first description was made in 1929 by Bedrna and Polcák in patients with chronic leukemia treated with radiotherapy. Later, its description in hematological malignancies increased and its association with lymphoma, leukemia and multiple myeloma is now widely recognized.3 For the purpose of diagnosis and classification, Cairo and Bishop divided the TLS into clinical and laboratory classifications (CTLS and LTLS). Patients are considered to have “laboratory tumor lysis syndrome” if two or more of the following metabolic changes occur: increase in serum levels of uric acid, potassium, or phosphate, or a decrease in calcium levels; or if there is a 25% increase in any of their levels over baseline within 3 days before, or 7 days after, the initiation of treatment.
Clinical tumor lysis syndrome is considered when a patient presents with LTLS plus one or more of the following complications: renal failure, arrhythmias / sudden death, or seizures. Likewise, depending on the type of neoplasm and the patient risk factors, the risk of developing TLS should be stratified as high, intermediate, or low. In high-risk cases, aggressive prophylaxis, including hydration, allopurinol, febuxostat, or rasburicase, is recommended.4,5 The impact of neurological, cardiac and renal complications is associated with poor prognosis and higher mortality. Hence, recognizing the risk of developing this life-threatening condition allows for prompt initiation of treatment. Despite the risk associated with the TLS, its diagnosis remains a challenge due to the complexity of hemato-oncological diseases and comorbidities of patients, in addition to the economic limitations in developing countries where patients tend to have less access to healthcare.
The incidence was described by Cairo et al. at 9.3% for LTLS and 6.7% for CTLS.6 However, in Brazil, the incidence and outcomes of the TLS have not been addressed and this study can help the scientific community understand the epidemiology of the TLS in our population, in which its prevention, diagnosis and treatment have become key to the evolution and improvement of treatments and targeted therapies.
MethodsStudy populationBetween January 2014 and December 2019, we retrospectively reviewed inpatients aged over 18 years with hematological neoplasms from the hematology service at the Hospital de Clinicas de Porto Alegre, a tertiary medical center with 1036 beds in southern Brazil. First, we conducted a screening focused on the identification of TLS based on laboratory criteria according to Bishop's definition. Then, we collected data using the Microsoft Excel software version 14.0 (Office 365) and imported it into the SPSS software version 21.0 (SPSS Inc. Chicago, IL) for data management and statistical analysis. Laboratory and clinical data were extracted from electronic medical records without identifying patient names and/or registries. The number of episodes was defined as a tumor lysis syndrome event in a hospital admission different from the previous episode.
The study was approved by the institutional ethical committee (approval number, 2020 - 0292) and was conducted in accordance with the Declaration of Helsinki.
Statistical analysisContinuous variables were described as median and interquartile range (IQR) for non-normally distributed variables and as mean and standard deviation (SD) for normally distributed data. Qualitative variables were described as absolute and relative frequency.
Means between groups were compared using the independent group t-test and the Mann–Whitney U test for normally and asymmetrically distributed data, respectively. The association between categorical variables was established using the Pearson chi-square test or the Fisher exact test. The estimations were made within a 95% confidence interval and the entire statistical tests were significant when the p-value was below the significance level of 0.05.
Variables that showed an effect on survival in univariate analyses (p < 0.10) were entered in multivariate models. The multivariate stepwise logistic regression analysis was performed with the Cox model and the overall survival (OS) was defined as the time from the neoplasm diagnosis to death or last follow-up date. The number of variables that could enter the multivariate analysis was limited using the N/10 rule to prevent overfitting the model. Survival outcomes were analyzed according to the Kaplan–Meier test.
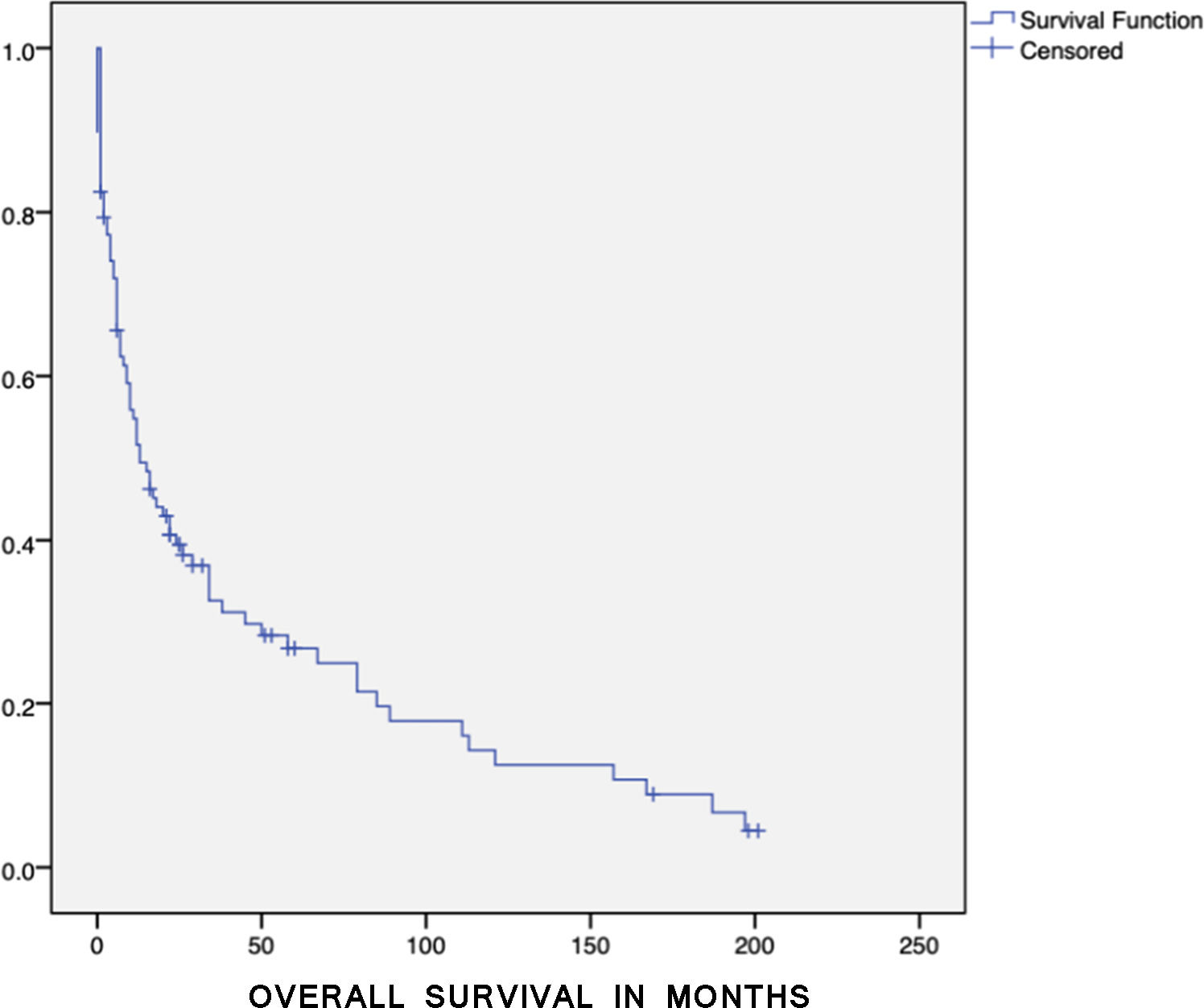
ResultsBetween 2014 and 2019, we evaluated the clinical and laboratory characteristics of TLS among the patients with hematological neoplasms from a tertiary and academic hospital in Brazil. Among these, 112 episodes in 97 patients were identified. Baseline characteristics are shown in Table 1. The incidence of TLS was 10.5% and the median OS was 13.0 months (95%CI 6.2 - 19.7) (Figure 1).
Baseline characteristics (n = 97).
| Variables | |
|---|---|
| Age at diagnosis | 56 (39.5 - 64) |
| Sex | |
| Female | 34 (35.1) |
| Male | 63 (64.9) |
| Performance status (ECOG) | |
| 0 | 20 (20.6) |
| 1 | 23 (23.7) |
| 2 | 28 (28.9) |
| 3 | 20 (20.6) |
| 4 | 6 (6.2) |
| Bulky disease at diagnosis* | |
| Yes | 11 (30.6) |
| No | 25 (69.4) |
| HIV | |
| Yes | 13 (13.4) |
| No | 84 (86.6) |
| HSCT | |
| Yes | 10 (10.3) |
| No | 87 (89.7) |
| Episodes number | |
| 1 | 83 (85.6) |
| 2 | 13 (13.4) |
| 3 | 1 (1.0) |
Data are n (%) or median (IQR).
Hematopoietic stem cell transplantation (HSCT).
The median age was 56 years (IQR 39.5 - 64); two-thirds were male and ten patients underwent hematopoietic stem cell transplantation (HSCT) previously. The most frequent diagnoses were multiple myeloma (MM) (18.6%), acute myeloid leukemia (AML) (17.5%), diffuse large B-cell lymphoma (DLBCL) (17.5%) and other high-grade B-cell lymphoma (10.3%) (Table 2). Concomitant HIV infection was found in 13.4% of the cases. Most of the lymphoma patients presented advanced disease (stages III and IV of the Ann Arbor Staging System). Eleven patients (30.6%) had bulky disease.
Tumor lysis syndrome according to disease.
Data are n (%).
About half of the patients required ICU admission (46.4%). Regarding the number of TLS episodes, most patients had only one (85.6%), 13.4% had two and 1% had three.
Within the diagnostic criteria for TLS, the most frequently altered variables were uric acid and phosphorus, median of 9.8 mg/dL (IQR 8.5 - 12) and 5.3 mg/dL (IQR 4.7 - 6.2), respectively (Table 3). All patients received intravenous (IV) hydration. The management also included the administration of allopurinol in 67% of the cases and rasburicase in eight patients. Renal replacement therapy was necessary in 37% of the cases (Table 4).
Laboratory values of biochemical parameters.
In the univariate analysis, the variables tested were sex, diagnosis, performance status, uric acid, creatinine, lactate dehydrogenase (LDH), renal replacement therapy, age, HIV status and ICU treatment. Age, HIV status and ICU treatment were significantly associated with OS. After the multivariate analysis, all of these variables remained significant. Overall mortality rate was 80.4% (n = 78) (Table 5).
DiscussionIn this study, we examined “real-world” data about the TLS in adult patients with hematologic malignancies at a single academic center over five years. We reported an incidence of 10.5%, consistent with the incidence described by other series of laboratory and clinical TLS (3% - 41%).6–9 The wide range of these data can be explained by differences in patient population, prophylaxis and treatment protocols.
It is remarkable that the most frequent diagnosis associated with the TLS was multiple myeloma (18.6%), despite its proliferation rate being low, in MM we have renal dysfunction as part of the clinical scenario, so these patients are also more susceptible to the TLS. However, in the post bortezomib era, there are descriptions of the rise in the tumor lysis syndrome. With an incidence ranging from 3.3% to 17.2%, the multivariate analyses revealed that the TLS was most strongly associated with bortezomib-containing therapy (odds ratio = 3.40, p = 0.069), followed by male sex (odds ratio = 2.29, p = 0.153), in a retrospective cohort study of 210 patients.10,11 Additionally, among the risk factors reported are extramedullary plasmacytoma, unfavorable cytogenetics, such as chromosome 13 deletion and elevated LDH.12,13 Proteasome inhibitors (PIs) have been the backbone in the treatment of MM. Its use remains common among our patient population, estimated at 38.8% (data not shown). Therefore, we believe that our results were influenced by the treatment, in addition to the risk factors previously described. Patients with hypocalcemia associated with the use of bisphosphonates were excluded to reduce bias. There were also no cases of MM with plasma cells in peripheral blood in our study.
In patients with AML, there are different prediction models of TLS, such as The Cairo, West Sussex and Hampshire Cancer Network (NHS), the Cancer and Leukemia Group B (CALGB) and serum uric acid (SUA) criteria-based,5,14–16 which include among their parameters, the white blood count (WBC), LDH, SUA, cytogenetic abnormalities and karyotype complexity. The incidence of LTLS has been reported between 17% and 26.4%.14,17 Interestingly, in our cohort, the highest SUA in AML was 14.3 mg/dL and Montesinos et al. found that a SUA > 7.5 mg/dL was associated with a 5.7-fold (95%CI 2.6 – 12.7) increased risk of LTLS. They also documented that only one-third of patients with LTLS criteria developed CTLS, that is, the form of TLS in which a higher induction mortality rate is observed.17 The treatment protocol during the study corresponded to conventional chemotherapy, that is, 3 + 7, FLAG-Ida, MEC and IC-APL 2006 (PETHEMA-based), except for one patient who was included in a clinical trial and also received a second-generation FLT3 inhibitor.
Our study also showed that, in the twenty-seven patients with DLBCL and other high-grade B-cell lymphoma, twelve cases were positive for HIV infection, which is a remarkable finding because this is a special population derived from the immunosuppression of HIV and the aggressiveness of the malignancy due to high tumor burden. Investigators at the Taipei Veterans General Hospital reported, in a cohort of 1200 patients living with HIV, 22 patients with NHL (3.7%), the DLBCL being the major histology subtype (63.6%), followed by Burkitt's lymphoma (BL) (36.4%). The TLS presented in 22.7% (5/22) of the patients and was identified as the only significant factor for very early mortality (OR: 11.3, 95%CI: 1.1 – 114.4, p = 0.04).18
Our multivariate analysis showed that for each year of age increase, the risk of mortality increases by 2% (HR 1.02; 95%CI [1.00 - 1.03], p = 0.002). Among HIV patients, the risk of mortality was three times higher (HR 3.19, 95%CI [1.71 - 5.93], p = < 0.005). Furthermore, the need for the ICU admission increased the risk of mortality by 76% (HR 1.76, 95%CI [1.16 - 2.69], p = 0.008), compared to patients who did not require the ICU. The multivariate analysis of other studies evaluated the risk factors for the development of the TLS, however, they did not assess the risk factors for the OS, as performed in our study.11,17 Furthermore, in our cohort, most patients (85.6%) had only one episode, 13.4% had two and 1% had three. Thus, our findings suggest that it is important to order baseline labs with frequent laboratory monitoring. To our knowledge, this is the first time in a retrospective cohort that these data were assessed.
Our study also showed that all patients received IV hydration, 67.9% used allopurinol and 7.1%, rasburicase. Thirty-seven percent of patients required dialysis; the number of sessions ranged from one to more than 50 sessions (data not shown). In our current medical practice, all patients diagnosed with hematologic malignancies receive prophylactic hydration and allopurinol.
Another issue is the economic impact. Annemans et al. reported, in a retrospective cohort with 788 acute leukemia and NHL including adults and pediatrics patients, that the average cost of management in patients with TLS is about 11 times higher than the average cost of managing patients with hyperuricemia not developing TLS (t-test on log-transformed cost, p < 0.0001). The main cost driver in TLS patients is the need for interventions (dialysis and hemofiltration), required in 25% of TLS patients, associated with admission to an intensive care unit.9
LimitationsThere are several limitations to this study. First, the retrospective data collection and single-institution experience are subject to bias. Second, the electrolyte disturbances from patients were evaluated to try to rule out other causes, such as the use of bisphosphonates in patients with MM. However, given the complexity of the treatment, it was not always possible to rule out confounding events.
ConclusionIn conclusion, TLS is a serious complication in the setting of hematological malignancies. The use of scores for risk stratification, as well as the implementation of prophylactic measures and prompt treatment with frequent laboratory monitoring are essential to reduce morbidity and mortality in the comprehensive treatment of these patients. Our data showed that the incidence of TLS is within that described in the international literature. Moreover, we have limited access to treatments, such as targeted therapy / novel agents, which can also trigger TLS. Therefore, this study can be used as reference to further studies that focus on such agents.
Financial disclosuresThe authors have no grants or reimbursement relevant to this article to disclose.