The clinical manifestation of foetal anaemia caused by maternal Kell alloantibodies differs from that caused by non-Kell alloantibodies. Severe anaemia develops in the foetus in the early weeks of gestation; therefore, proper management and early intervention are important. A systematic review and meta-analysis was performed to determine whether the anti-K1 titre can determine the sequelae of Kell alloimmunised pregnancies. Prospective and retrospective cohort studies were used to conduct a systematic review following a comprehensive literature search, in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Studies were screened based on a defined set of inclusion and exclusion criteria. A total of 5143 potential articles were identified. Ten studies were used in the meta-analysis of pregnancy outcomes for a specific anti-K1 titre cut-off. The meta-analysis identified statistical significance for intrauterine transfusion (ARD: 0.351; 95 % CI: 0.593–0.109; p-value = 0.004), hydrops (ARD: 0.808; 95 % CI: 1.145–0.472; p-value <0.001), intrauterine foetal death (ARD: 0.938; 95 % CI:1.344 to -0.533; p-value <0.001) and intrauterine transfusion for Doppler middle cerebral artery >1.5 MoM (ARD: 0.381; 95 % CI:1.079 to -0.317; p-value = 0.285). It was concluded that there is no correlation between anti-K1 titre and Kell sensitised pregnancy outcomes, but monitoring the anti-K1 titre is important to manage the pregnancy and it helps clinicians determine the need for intrauterine transfusions. Doppler middle cerebral artery peak systolic velocity is strongly correlated with foetal anaemia and is an efficient routine method for determining the need for intrauterine transfusions in pregnancies affected by anti-K1.
The Kell blood group system is a complex blood group system and contains 36 high prevalence and low prevalence antigens. The K1 antigen is the third most immunogenic antigen (5 %) after the ABO and D antigens. The K antigen has a low prevalence.1
The Kell (K1) antigen was discovered in 1945 and named after a pregnant woman who developed Kell alloantibodies named Mrs Kelleher; her firstborn suffered from severe anaemia and was found to be Kell positive. Anti-K1 is an IgG1 antibody that is formed after sensitisation through transfusion or pregnancy. Naturally occurring IgM antibodies are rare. Anti-K1 is associated with severe haemolytic transfusion reactions and haemolytic disease of the foetus and newborn. Maternal alloantibodies may cause anaemia in Kell-positive foetuses. Maternal anti-K1 are formed by transfusion, or the mother can also develop anti-K1 alloantibodies during a previous or current pregnancy.2
Foetal anaemia associated with anti-K1 develops by two mechanisms: haemolysis of foetal red blood cells and suppression of erythropoiesis.3
Foetal erythrocytes are haemolysed by maternal antibodies (IgG1) which cross the placenta and attach to foetal blood cells. These sensitised RBCs pass through the foetal spleen and filter out to the blood and reticuloendothelial system, where they are destroyed and free haemoglobin results in increased levels of bilirubin. There is a compensatory increase in erythropoiesis and an increase in the number of reticulocytes.
Suppression of erythroid progenitor cells by anti-K1The Kell protein is a zinc endopeptidase that converts endothelin-3 to an active vasoconstrictor. The Kell antigen is present on immature erythroid progenitor cells in early foetal life at 10–11 weeks of gestation.4 It is believed that Kell proteins are believed to play a role in RBC growth and differentiation.5
HDFN associated with maternal anti-K1 may result in severe foetal anaemia, foetal hydrops, asphyxia, and perinatal death. Hyperbilirubinemia can lead to kernicterus and neurological disabilities in newborns. Unfortunately, a foetus with anti-K1 can develop hydrops during early pregnancy, hydrops can develop at <20 weeks of gestation.6 Severe foetal anaemia should be detected before the development of hydrops.
A study by Lindenburg et al. revealed that the occurrence of anti-K1 was significantly higher (24 %) in pregnancies requiring intrauterine transfusions (IUTs) prior to reaching 20 weeks compared to transfusions after 20 weeks (11 %).7
Studies have shown that the survival rate of RhD blood group incompatibility is 89 %, which is higher than the HDFN caused by anti-K1 (58 %); the difference is due to delayed diagnosis. Administration of anti-D antibodies during pregnancy also decreased the RhD incidence of Rh D alloimmunisation.
The current management of pregnancies complicated by maternal Kell (K1) antibodies includes antibody screening. Antibodies are identified during the indirect antiglobulin test phase. Early antibody screening can help improve the pregnancy outcome,8 with the next step after confirming the presence of an antibody being to identify the fatherʼs zygosity. If the father is heterozygous (K, k), foetal Kell typing is performed through amniocentesis. In rare cases, if the father is homozygous (K, K), antibody titration is performed without foetal Kell typing. Heterozygous K-positive cells (single doses) are used for the antibody titration. Two registered technologists typically determine the titres to ensure agreement.9 There are many observations to determine the threshold values for anti-K1 titres. Since the disease occurs with low titres, many studies have concluded that there is no correlation between titres and pregnancy outcome.8 The amniotic fluid bilirubin test is not very helpful and does not correlate with the severity of foetal anaemia. In one study, a threshold of 1:2 was used, which was monitored from 17 weeks onwards; when the tires were higher than the threshold, a middle cerebral artery (MCA) ultrasound was performed to measure the peak systolic volume to evaluate the PSV.4 If the multiples of the median (MoM) is <1.5, Doppler ultrasound of the MCA is repeated after 1–2 weeks. If MoM >1.5 or hydrops is present, foetal blood sampling was performed. Haematological parameters help clinicians decide the need for IUT. Antibody titres and Doppler ultrasound help determine the necessity of foetal blood sampling and the need for intervention.
The risk of developing severe anaemia and hydrops in pregnancies with anti-K1 can be predicted by non-invasive investigations.
Foetal blood sampling is the gold standard test to detect foetal anaemia, but it carries a foetal loss rate of 1.4 % per procedure.10
This study aimed to explore the effectiveness of non-invasive investigation, anti-K1 titre, and MCA ultrasound before cordocentesis in detecting high-risk pregnancies and improving perinatal outcomes.
Material and methodsStudy designThe meta-analysis followed the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) protocol to explore and identify studies investigating the role of anti-K1 titres and peak systolic velocity (PSV) of the foetal MCA in predicting the severity of foetal anaemia and hydrops, and the need for IUT in Kell alloimmunised pregnancies.11 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to ensure the quality of the included studies.12
Search strategyFor this meta-analysis, relevant literature was obtained using an electronic database and manual search. PubMed, Embase, and Science Direct databases were systematically searched from January 2000 to June 2022. Google Scholar was manually searched. The same search terms, phrases, and test descriptions were used for both manual and electronic searches. The keywords, phrases and test descriptions included: “haemolytic disease of the foetus and newborn”, “anti-K1 titre”, “Kell alloimmunised pregnancies”, “Maternal Kell alloantibody”, “hydrops”, “intrauterine transfusion”, “MCA Doppler ultrasound”, “middle cerebral artery peak systolic velocity”, “hyporegenerative anaemia”, “hyperbilirubinemia” and “Decreased reticulocytes in Kell alloimmunised pregnancies”. Furthermore, some references in the articles were identified as appropriate studies.
Study selection and eligibility criteriaArticles were identified using the search strategy and screened using the designated exclusion and inclusion criteria to obtain the eligible studies for the meta-analysis. After scrutinising the titles and abstracts, the full manuscript of potentially eligible articles was obtained. Some studies were manually selected based on predefined criteria. Articles with cell-alloimmunised pregnancies were selected. Studies were included if data on the anti-K1 cut-off titre, foetal anaemia, hydrops, intrauterine foetal death (IUFD) and MCA Doppler ultrasound for PSV could be extracted. The articles included were observational, retrospective, or prospective cohort studies.
Systematic reviews, case studies, literature reviews, and conference proceedings were excluded. Articles in languages other than English and those with unavailable translations were excluded.
Some studies were excluded because their full text was not available. Moreover, articles from which data related to Kell alloimmunised pregnancies could not be extracted were excluded.
Assessment of quality and bias in included studiesThe quality of all articles was assessed in terms of population, intervention, and outcomes. The eligible studies were evaluated for methodological quality and bias using published standards outlined by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.12
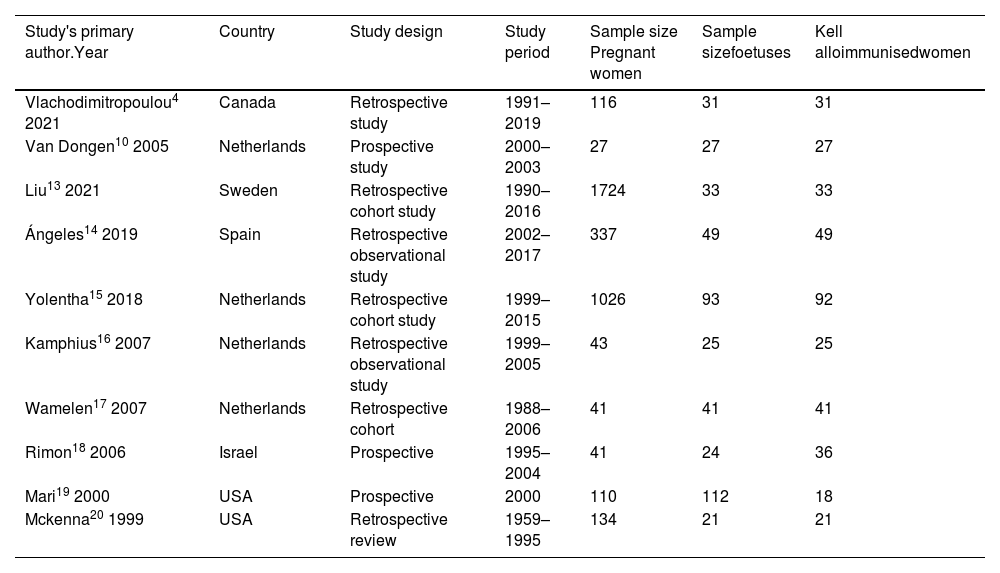
Data extractionThe information obtained from each study is summarised in the tables. The study characteristics and sample sizes tabulated are shown in Table 1. Table 2 illustrates the outcomes of anti-K1 sensitised pregnancies using anti-K1 titre cut-off values as a non-invasive test. The consequences of Kell-sensitised pregnancies using PSV of the foetal MCA as a non-invasive procedure above and equal to the cut-off value of 1.5 MoM are listed in Table 3.
Study characteristics and sample size.
| Study's primary author.Year | Country | Study design | Study period | Sample size Pregnant women | Sample sizefoetuses | Kell alloimmunisedwomen |
|---|---|---|---|---|---|---|
| Vlachodimitropoulou4 2021 | Canada | Retrospective study | 1991–2019 | 116 | 31 | 31 |
| Van Dongen10 2005 | Netherlands | Prospective study | 2000–2003 | 27 | 27 | 27 |
| Liu13 2021 | Sweden | Retrospective cohort study | 1990–2016 | 1724 | 33 | 33 |
| Ángeles14 2019 | Spain | Retrospective observational study | 2002–2017 | 337 | 49 | 49 |
| Yolentha15 2018 | Netherlands | Retrospective cohort study | 1999–2015 | 1026 | 93 | 92 |
| Kamphius16 2007 | Netherlands | Retrospective observational study | 1999–2005 | 43 | 25 | 25 |
| Wamelen17 2007 | Netherlands | Retrospective cohort | 1988–2006 | 41 | 41 | 41 |
| Rimon18 2006 | Israel | Prospective | 1995–2004 | 41 | 24 | 36 |
| Mari19 2000 | USA | Prospective | 2000 | 110 | 112 | 18 |
| Mckenna20 1999 | USA | Retrospective review | 1959–1995 | 134 | 21 | 21 |
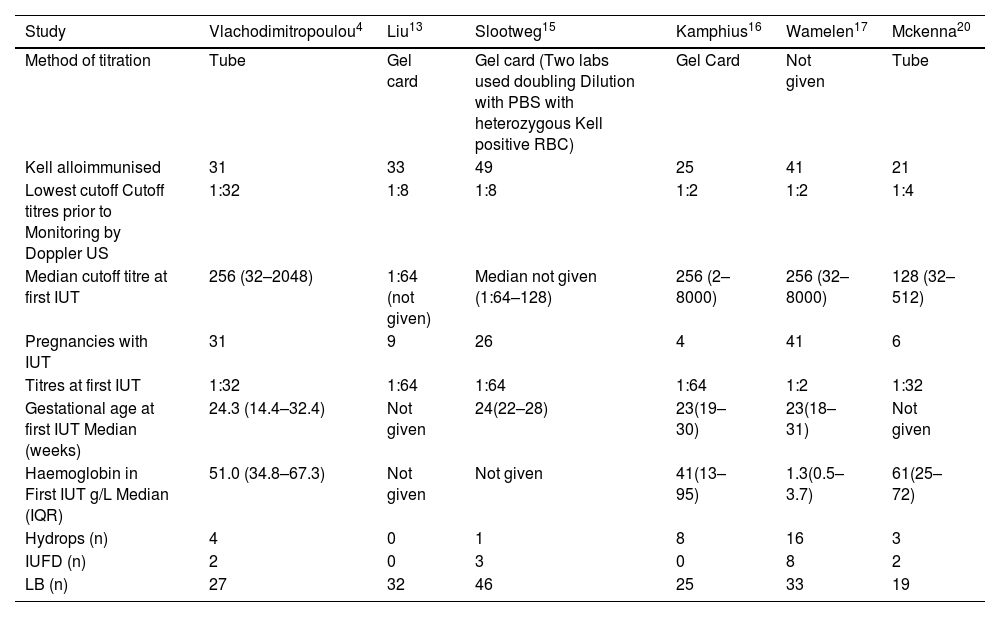
The outcomes of anti-Kell sensitised pregnancies using Kell antibody titre cutoff values as non-invasive test.
| Study | Vlachodimitropoulou4 | Liu13 | Slootweg15 | Kamphius16 | Wamelen17 | Mckenna20 |
|---|---|---|---|---|---|---|
| Method of titration | Tube | Gel card | Gel card (Two labs used doubling Dilution with PBS with heterozygous Kell positive RBC) | Gel Card | Not given | Tube |
| Kell alloimmunised | 31 | 33 | 49 | 25 | 41 | 21 |
| Lowest cutoff Cutoff titres prior to Monitoring by Doppler US | 1:32 | 1:8 | 1:8 | 1:2 | 1:2 | 1:4 |
| Median cutoff titre at first IUT | 256 (32–2048) | 1:64 (not given) | Median not given (1:64–128) | 256 (2–8000) | 256 (32–8000) | 128 (32–512) |
| Pregnancies with IUT | 31 | 9 | 26 | 4 | 41 | 6 |
| Titres at first IUT | 1:32 | 1:64 | 1:64 | 1:64 | 1:2 | 1:32 |
| Gestational age at first IUT Median (weeks) | 24.3 (14.4–32.4) | Not given | 24(22–28) | 23(19–30) | 23(18–31) | Not given |
| Haemoglobin in First IUT g/L Median (IQR) | 51.0 (34.8–67.3) | Not given | Not given | 41(13–95) | 1.3(0.5–3.7) | 61(25–72) |
| Hydrops (n) | 4 | 0 | 1 | 8 | 16 | 3 |
| IUFD (n) | 2 | 0 | 3 | 0 | 8 | 2 |
| LB (n) | 27 | 32 | 46 | 25 | 33 | 19 |
PBS: Phosphate-buffered saline; RBC: Red blood cells; US: Ultrasound; IUFD: Intrauterine foetal death; IUT: Intrauterine transfusion; IQR: Inter quartile range.
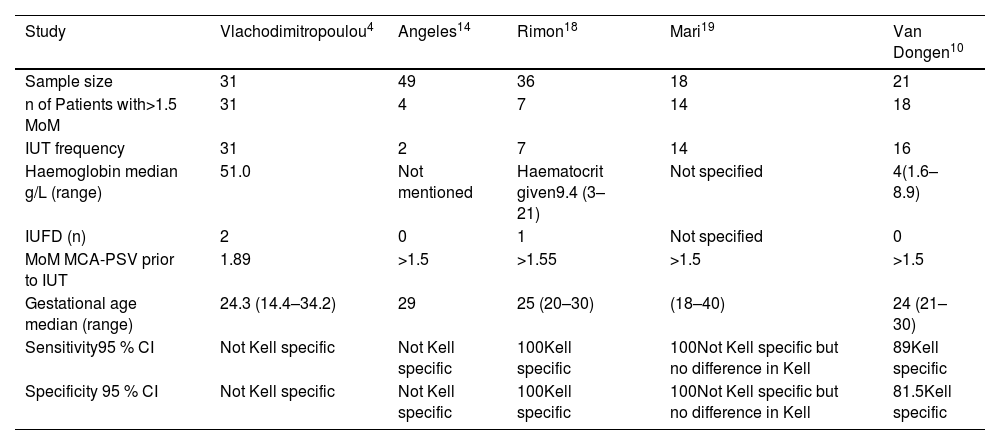
Results of Kell sensitised pregnancies using peak systolic velocity of foetal middle cerebral artery as a non-invasive procedure≥1.5 MoM.
| Study | Vlachodimitropoulou4 | Angeles14 | Rimon18 | Mari19 | Van Dongen10 |
|---|---|---|---|---|---|
| Sample size | 31 | 49 | 36 | 18 | 21 |
| n of Patients with>1.5 MoM | 31 | 4 | 7 | 14 | 18 |
| IUT frequency | 31 | 2 | 7 | 14 | 16 |
| Haemoglobin median g/L (range) | 51.0 | Not mentioned | Haematocrit given9.4 (3–21) | Not specified | 4(1.6–8.9) |
| IUFD (n) | 2 | 0 | 1 | Not specified | 0 |
| MoM MCA-PSV prior to IUT | 1.89 | >1.5 | >1.55 | >1.5 | >1.5 |
| Gestational age median (range) | 24.3 (14.4–34.2) | 29 | 25 (20–30) | (18–40) | 24 (21–30) |
| Sensitivity95 % CI | Not Kell specific | Not Kell specific | 100Kell specific | 100Not Kell specific but no difference in Kell | 89Kell specific |
| Specificity 95 % CI | Not Kell specific | Not Kell specific | 100Kell specific | 100Not Kell specific but no difference in Kell | 81.5Kell specific |
MoM: Middle of median; MCA: Mid Cerebral artery; IUT: Intrauterine transfusion; PSV: Peak systolic velocity; CI: Confidence interval.
A forest plot for the meta-analysis was constructed from data in Tables 3 & 4 by using Open Meta-Analyst software (version 4.06.15) downloaded from the Brown University website. Two-armed proportion analysis was used to express the Arcsine risk difference and 95 % confidence interval using binary random effect model with maximum likelihood. A forest plot was constructed with 95 % confidence intervals, p-values and heterogeneity. A p-value ≤ 0.05 was considered statistically significant.
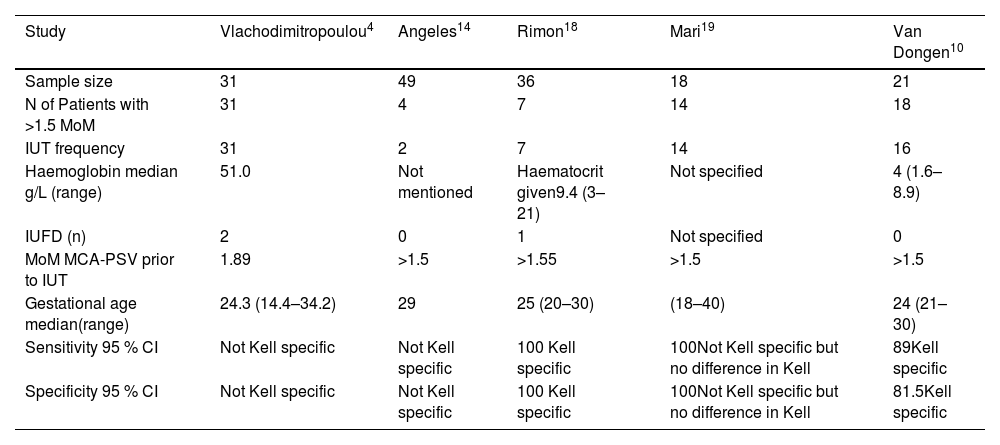
Results of Kell sensitised pregnancies using peak systolic velocity of foetal middle cerebral artery as a non-invasive procedure≥1.5 Mom.
| Study | Vlachodimitropoulou4 | Angeles14 | Rimon18 | Mari19 | Van Dongen10 |
|---|---|---|---|---|---|
| Sample size | 31 | 49 | 36 | 18 | 21 |
| N of Patients with >1.5 MoM | 31 | 4 | 7 | 14 | 18 |
| IUT frequency | 31 | 2 | 7 | 14 | 16 |
| Haemoglobin median g/L (range) | 51.0 | Not mentioned | Haematocrit given9.4 (3–21) | Not specified | 4 (1.6–8.9) |
| IUFD (n) | 2 | 0 | 1 | Not specified | 0 |
| MoM MCA-PSV prior to IUT | 1.89 | >1.5 | >1.55 | >1.5 | >1.5 |
| Gestational age median(range) | 24.3 (14.4–34.2) | 29 | 25 (20–30) | (18–40) | 24 (21–30) |
| Sensitivity 95 % CI | Not Kell specific | Not Kell specific | 100 Kell specific | 100Not Kell specific but no difference in Kell | 89Kell specific |
| Specificity 95 % CI | Not Kell specific | Not Kell specific | 100 Kell specific | 100Not Kell specific but no difference in Kell | 81.5Kell specific |
MoM: Middle of median; MCA: Mid Cerebral artery; IUT: Intrauterine transfusion; PSV: Peak systolic velocity; CI: Confidence interval.
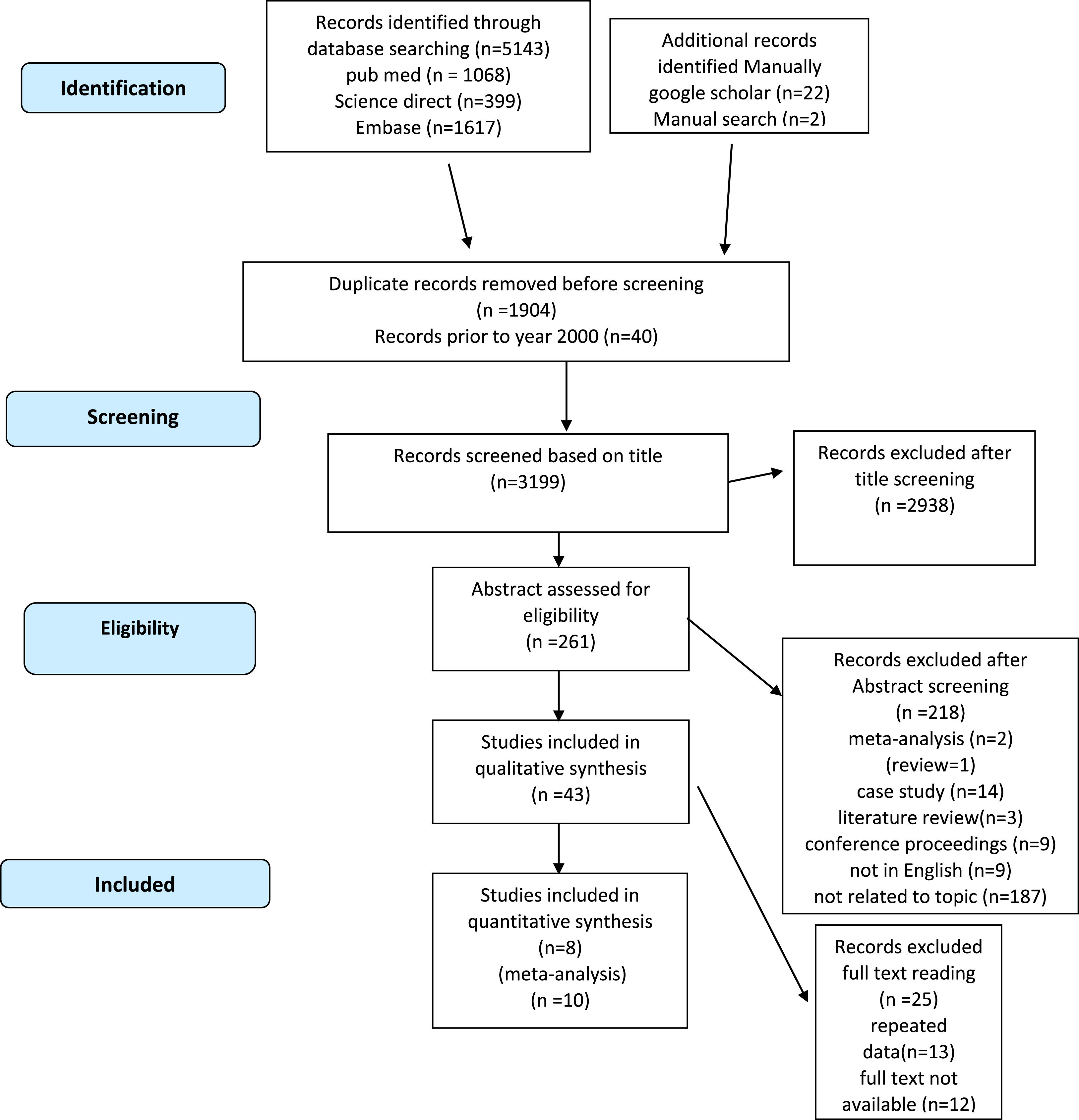
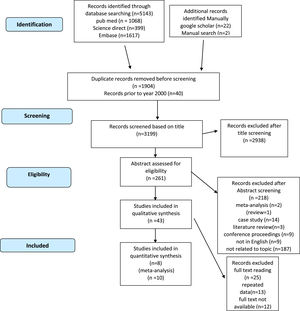
Initially, 5143 potential articles were identified in PubMed, Science Direct, Scopus and Embase. In addition, 24 potential articles were identified through a manual search of Google Scholar. After removing duplicates, the titles of 3223 studies were screened. Most (2962 titles) were found to be irrelevant to the topic. Furthermore, 218 studies were excluded after screening the abstracts. The full texts of 42 articles were thoroughly explored. Twenty-five studies were excluded after reading the full texts. Ultimately, 18 studies were included in the analyser and were pooled for systematic review. As eight of them lacked sufficient data for quantitative analysis, they were excluded from the study and thus ten articles were found to have relevant data for the meta-analysis (Figure 1).
Characteristics of eligible studiesThe included studies were retrospective cohort studies4,13–17 and prospective studies.10,18–20 The citations included studies from six different countries from 1999 to 2021. The sample included Kell-alloimmunised pregnant women and Kell-positive foetuses. In all studies, there were singleton foetuses except for one study that had one twin pregnancy.15Table 1 summarises the characteristics of the studies.
Anti-K1 titre monitoring to detect the severity of the diseaseThe roles of monitoring anti-K1 titres in six articles are summarised in Table 2.4,13,15–17,20 The titration methods are described below. In one study, this technique was not specified.17 In each eligible study, the lowest cut-off titre used to monitor pregnancy by foetal ultrasound of the middle cerebral artery was specified. Additionally, anti-K1 titres at the first IUT were outlined in each study. The number of Kell alloimmunised women who underwent IUT with the median gestational age at which they were transfused is also specified in Table 2. The median haemoglobin level at which the first IUT was performed was also extracted from the articles. In two studies, the median haemoglobin value was not determined.13,15 The outcomes of Kell-alloimmunised pregnancies are shown in Table 3. The number of cases with hydrops in each study, intrauterine deaths and live births in Kell-alloimmunised pregnancies are included.
Foetal ultrasound middle cerebral artery-peak systolic velocity to detect the severity of the diseaseThe frequency of IUTs in Kell-sensitised pregnancies with median gestational age when PSV of the foetal MCA ≥1.5 multiple of medians (MoM) is summarised in Table 3. The results were obtained from five eligible studies.4,10,14,16,18 Median PSV (cm/s) was given in three studies.4,18,10 Two studies also mentioned the anti-K1-specific sensitivity and specificity of MCA Doppler ultrasound for peak systolic velocities.10,18
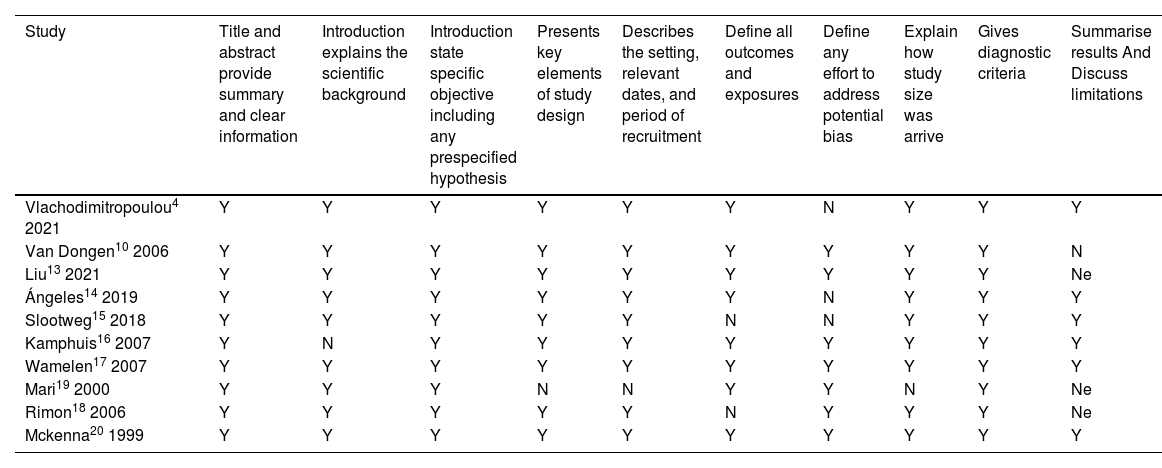
Study quality assessmentStrobe criteria were used to assess the quality of the eligible studies. The most relevant criteria are listed in Table 4. Most of the studies were high-quality studies and fulfilled the criteria for methodological quality assessment; however, in two articles, limitations were not discussed.13,19 One study failed to discuss all outcomes.18 In three articles, efforts to address potential bias were not defined.4,14,15 Most of the studies had high methodological quality, which suggests that the risk of bias through irrelevant articles is low (Table 5).
Assessment of included study's methodological quality according to strobe checklist. Y: yes; N: No; Ne: Limitation not discussed.
| Study | Title and abstract provide summary and clear information | Introduction explains the scientific background | Introduction state specific objective including any prespecified hypothesis | Presents key elements of study design | Describes the setting, relevant dates, and period of recruitment | Define all outcomes and exposures | Define any effort to address potential bias | Explain how study size was arrive | Gives diagnostic criteria | Summarise results And Discuss limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| Vlachodimitropoulou4 2021 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Van Dongen10 2006 | Y | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Liu13 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Ne |
| Ángeles14 2019 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Slootweg15 2018 | Y | Y | Y | Y | Y | N | N | Y | Y | Y |
| Kamphuis16 2007 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Wamelen17 2007 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Mari19 2000 | Y | Y | Y | N | N | Y | Y | N | Y | Ne |
| Rimon18 2006 | Y | Y | Y | Y | Y | N | Y | Y | Y | Ne |
| Mckenna20 1999 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
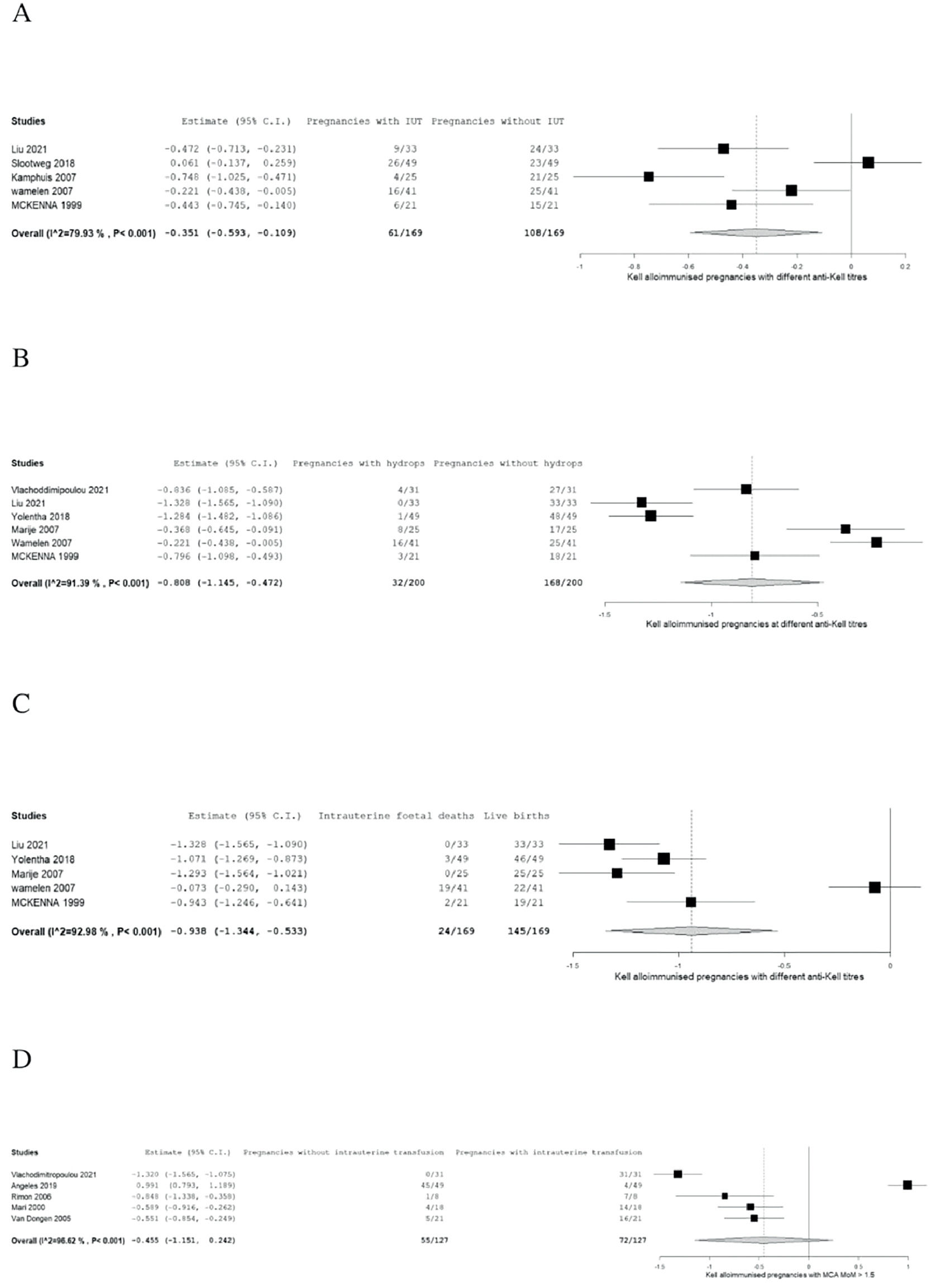
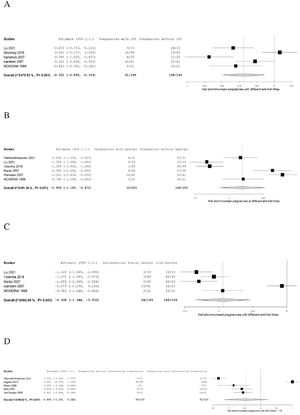
The proportion of IUT, hydrops foetalis and IUFD are summarised in forest plots in Figure 2A–C based on six included citations.4,13,15,17,20 Specific cut-off values of anti-K1 titres before monitoring by Doppler ultrasound and before IUT were determined in the studies, as shown in Table 3.
A: Forest plot of meta-analysis of number of Kell alloimmunised pregnancies with and without intrauterine transfusions at different cutoff antibody titres. B: Forest plot of meta-analysis of number of Kell alloimmunised pregnancies of newborns with and without hydrops at different cutoff antibody titres. C: Forest plot of meta-analysis of number of Kell alloimmunised pregnancies with and without intrauterine foetal deaths at different cutoff antibody titres. D: Meta-analysis of intrauterine transfusion for Doppler ultrasound middle cerebral artery multiples of the median ≥1.5.
The Forest plots were interpreted using two-arm proportion analysis which was expressed in Arcsine risk difference 95 % confidence interval (95 % CI) using binary random effect model with maximum likelihood.
Meta-analysis on intrauterine transfusionThe numbers of pregnant women with and without IUT in Kell alloimmunised pregnancies at different anti-K1 cut-off titres and gestational weeks, as shown in Table 2, were analysed using a forest plot.
The result of the meta-analysis on IUT was found to be statistically significant (absolute risk difference - ARD: 0.351 95 % CI: −0.593 to −0.109; p-value = 0.004); however, high heterogeneity can be seen as the study has variable outcomes (p-value <0.001 and I2value = 79.928) as interpreted by the forest plot in Figure 2A.
Meta-analysis on hydropsThe proportion of pregnancies with and without hydrops is summarised in a forest plot (Figure 2B). This result was statistically significant (ARD: 0.808; 95 %CI −1.145 to −0.472; p-value <0.001). Heterogeneity is high as there are variable outcomes. (p-value <0.001 and I2 = 92.802).
Meta-analysis on intrauterine foetal deathThe alloimmunised pregnancies that resulted in live births and those that resulted in IUFD are summarized in a forest plot (Figure 2C). The results of the meta-analysis of IUFD proved its statistically significance (ARD: −0.938; 95 % CI: −1.344 to −0.533; p-value <0.001) but the heterogeneity was very high (p-value <0.001 and I2 = 92.976).
Meta-analysis on intrauterine transfusion for Doppler ultrasound of the middle cerebral artery multiples of the median ≥1.5Meta-analysis was performed for the number of pregnancies with PSV >1.5 MoM and PSV <1.5 MoM. Table 3 shows the number of IUT in different studies of pregnancies with PSV >1.5 MoM.
The forest plot (Figure 2D) shows that the results were not statistically significant (ARD: 0.381; 95 % CI: −1.079 to −0.317; p-value = 0.285) with high heterogeneity (p-value <0.001 and I2 = 97.748).
DiscussionFoetal anaemia associated with maternal Kell alloantibody is as severe as anaemia caused by anti-RhD. 21 As already discussed, Kell mediated foetal and neonatal anaemia is caused by haemolysis and destruction of erythroid progenitor cells; therefore, foetal anaemia caused by anti-K1 is a consequence of two mechanisms. Therefore, haematological parameters, antibody titre threshold values, clinical features, management, and perinatal and postnatal interventions are different from foetal and neonatal anaemia caused by non-Kell alloimmunised pregnancies. In these studies, high heterogeneity was observed, which was due to the variable outcomes of Kell alloimmunised pregnancies.
Monitoring pregnancies complicated by anti-K1Foetal perinatal and postnatal reticulocytes count in Kell sensitised pregnanciesIt was observed that the foetuses affected by Kell alloantibody had fewer circulating reticulocytes than the classic haemolytic disease of the foetus and newborn caused by anti-D.22
In 1994, it was observed that the severity of anaemia and the development of hydrops foetalis are not related to the absence of reticulocytosis or erythroblastosis. It was also observed that unlike the foetus affected by anti-D, there was no linear correlation between foetal haematocrit and circulating reticulocytes. Thus, it was concluded that the mechanism involved was the suppression of erythroid cells.21
In 1996, another study commented on the plasma level of erythropoietin in foetuses affected by haemolytic disease. It was found that the foetal mean plasma erythropoietin level was higher in foetuses with anti-K1 than in foetuses with anti-D. This observation led to the conclusion that there was an inadequate erythroid stem cell response to erythropoietin.9
Bilirubin levels in the neonates with anaemia caused by Kell antibodyBefore 2000, foetal amniotic bilirubin, measured by an invasive procedure, was used to determine the need for IUT; however, non-invasive MCA Doppler ultrasound has now replaced this method.
Foetal bilirubin levels were compared between pregnancies complicated by different alloantibodies. It was found that the bilirubin level at the first IUT in Kell (K1) alloimmunised pregnancies was lower than that in non-Kell-alloimmunised pregnancies.23–25 In another study, it was stated that although the bilirubin level in neonates with anti-K1 was higher than the reference range, it was lower than the serum bilirubin level in neonates with Anti-D. It was suggested that the dominant mechanism of anaemia was the suppression of erythroid cells.4
Anti-K1 titresAfter assessing the data from six studies showing that in pregnancies mediated by anti-K1, the antibody titres were low. Different authors have suggested different anti-K1 titre threshold values for prenatal monitoring and IUT.4,13,15–17,20
Through this meta-analysis, we found that there is no correlation between anti-K1 titres and disease severity. Hence, there was no correlation between antibody titres and the development of hydrops or IUFD.
In contrast, most studies have shown that anti-K1 titres correlate with the number of IUTs in each pregnancy. In one study, the threshold value of the anti-K1 titre was considered to be 1:4. It was observed that 60 % of pregnancies with titres >1:4 required either IUT or transfusions after birth.15
In a study conducted in 2007, Wamelen et al discussed a case with severe foetal anaemia with an antibody titre of 1:2 and suggested that close monitoring of Kell sensitised pregnancies with titres above 1:2 is the safest approach.17
In two studies, the authors suggested that the mean number of IUT per pregnancy can be calculated using the anti-K1 titre.4,17
It was concluded that the lower the titre at the time of IUT, the higher the gestational age, so it is possible to say that if the titre remains low throughout pregnancy, then it can be predicted that the prognosis of the disease is good.4,15–17
Although the severity of the disease was not related to the lowest cut-off value, it was found in one study that the foetus developed hydrops at an anti-K1 titre of 1:8 in the 20th week of gestation.25
In the eligible studies, the lowest cut-off titre at which pregnancy should be monitored weekly was reported as 1:2 in the 18th week of gestation.16,17
In Kell (K1), alloimmunised pregnancy foetuses present with severe anaemia at early gestational age therefore timely detection and early management is important for a favourable outcome.
It is also noted that continuously observing the K1 antibody titre levels and detecting an increase in titre can indicate a negative outlook for the disease and it is helpful for clinicians to decide the type of intervention needed.
Doppler ultrasound of the middle cerebral artery/peak systolic velocityAfter assessing the data from five eligible studies, it was observed that MCA ultrasound >1.5 MoM in an early gestational period predicted unfavourable perinatal outcomes.4,10,18,19
As we have already discussed, severe foetal disease is associated with low anti-K1 titres and early monitoring is important in deciding the intervention. Therefore, Doppler ultrasound is used as an efficient follow-up test. It was observed that 53 % of Kell alloimmunised pregnancies were routinely investigated using MCA Doppler ultrasound.14
Foetal blood sampling was used to investigate pregnancy to determine whether any further intervention was required however, foetal blood sampling has a 1 % risk of foetal loss. Consequently, Doppler ultrasound MCA-PSV is safer and cheaper.18
Although Doppler ultrasound is regarded as the most reliable method to predict severe anaemia in Kell alloimmunised pregnancies, its reliability decreases after 35 weeks of gestation and can result in false negative results.14
In two studies, it was found that the sensitivities of Doppler MCA in detecting anaemia in Kell alloimmunised pregnancies were 100 % and 89 % and the specificities were 100 % and 87 %.10,18
Interventions in Kell alloimmunised pregnanciesIUT should be performed before the development of hydrops foetalis. Even though the procedure has a lot of complications that can lead to neonatal death, it has been observed that it can prevent the development of hydrops in Kell alloimmunised pregnancies.8
It has been reported that some neonates with hyperbilirubinemia require phototherapy and exchange transfusion due to haemolytic anaemia, but the need of phototherapy and exchange transfusion is lower than in cases of HDFN caused by anti-D.20
LimitationsThis review has some limitations. In this study, it was not specified whether the alloimmunisation was due to a previous pregnancy, current pregnancy, or transfusion induced. Obstetric history of Kell alloimmunised pregnant women was not mentioned. The study period in some studies was long and the techniques and criteria for IUT have changed. Post-natal investigations and interventions are not included.
The meta-analysis of the number of patients with and without IUT with MCS/PSV >1.5 MoM shows that the results were not statistically significant, which may be due to the small sample size in the included studies.
ConclusionMaternal Kell alloantibodies cause severe disease in the foetus and newborn in the early weeks of gestation therefore, early management and proper monitoring are required.
The Australian and New Zealand Society of Blood Transfusion (ANZSBT) recommends anti-K1 titre monitoring every four weeks until the 28th week of gestation. After 28 weeks of gestation, weekly antibody titre monitoring is recommended in foeto-maternal units. Pregnancies are monitored by MCA ultrasound to prevent HDFN by Kell alloimmunisation, withholding Kell negative units for females is recommended.26
CRediT authorship contribution statementMufleha Ahmed: Conceptualization, Methodology, Data curation, Writing – original draft. Denise E. Jackson: Conceptualization, Supervision, Writing – review & editing.
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.