Sickle cell disease (SCD) comprises a heterogeneous group of inherited hemolytic disorders that increases the risk of maternal and perinatal complications due to chronic systemic inflammatory response, endothelial damage and vaso-occlusion. The contribution of genotypes to the severity of outcomes during pregnancy is not completely established.
MethodsA retrospective study of medical charts was performed to compare maternal and perinatal outcomes in Hb SS, Hb SC disease and sickle-beta thalassemia (Hb Sβ) pregnancies followed at a high-risk antenatal care unit over a 6-year period. A descriptive analysis of morphological findings was performed of the placenta when pathology reports were available.
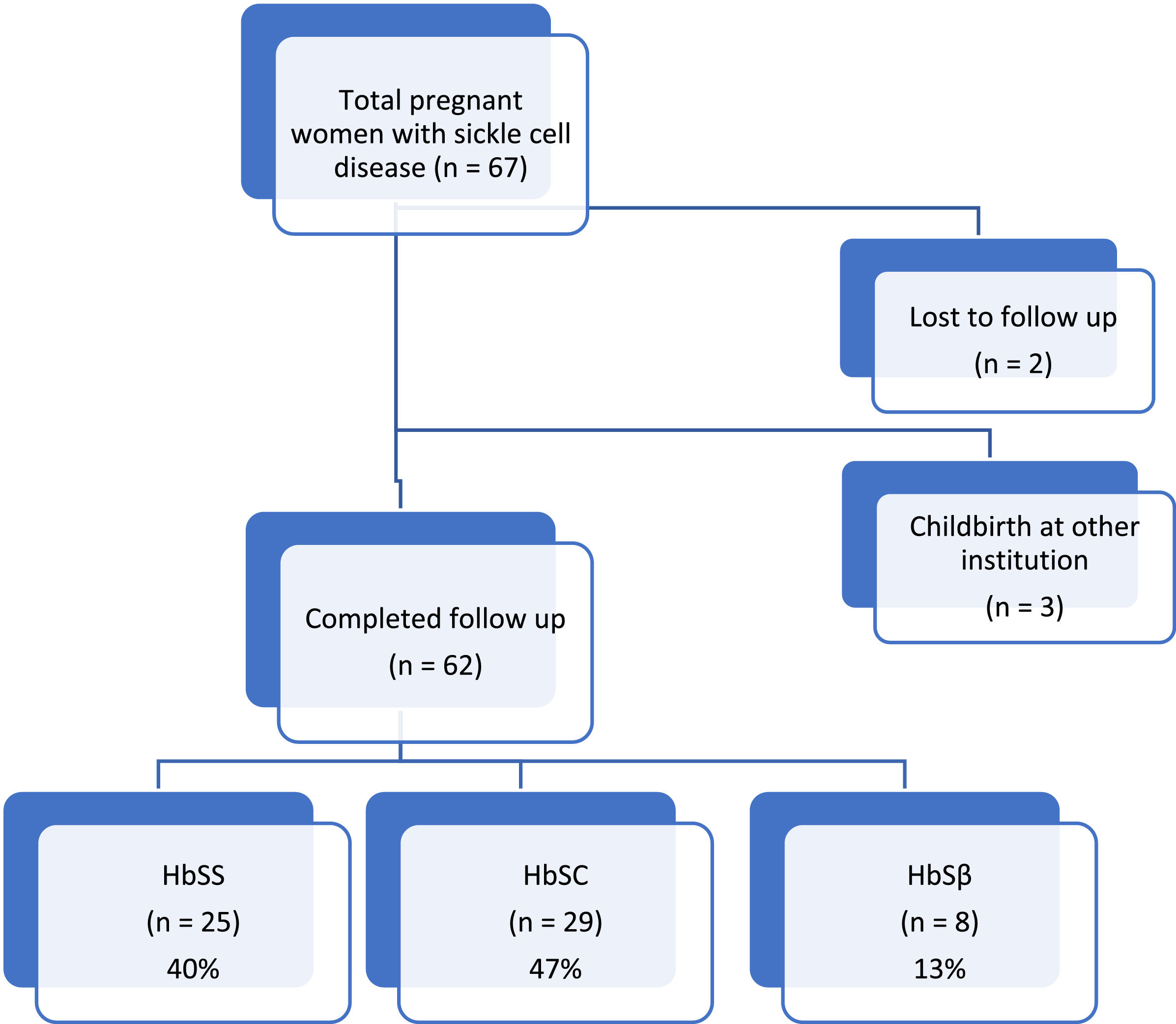
ResultsSixty-two SCD pregnant women [25 Hb SS (40 %), 29 Hb SC (47 %) and 8 Hb Sβ (13 %)] were included. Overall, SCD was associated with maternal complications (77 %), preterm birth (30 %), cesarean section (80 %) and a need of blood transfusion. In general there were no statistically significant differences between genotypes. The only significant difference was the hemoglobin level at first antenatal care visit which was lower for the homozygous genotype (7.7 g/dL) compared to Hb SC and Hb Sβ (9.7 g/dL and 8.4 g/dL, respectively; p-value = 0.01). Ten of 15 evaluated placentas showed abnormal morphological findings
ConclusionSCD, regardless of the underlying genotype, is associated with increased adverse maternal and perinatal outcomes and placental abnormalities associated with maternal vascular malperfusion.
Sickle cell disease (SCD) is one of the most prevalent recessive inherited diseases worldwide1 affecting more than 300,000 newborns every year.2-4 In Brazil, more than 2 million people carry the sickle cell gene mutation and about 25–50 thousand people live with homozygote sickle cell anemia (Hb SS).5
The structurally abnormal hemoglobin S (Hb S) is the result of a single amino acid substitution in the HBB gene encoding the β-globin chain. Deoxy-Hb S polymerizes at low oxygen levels, stretching the red blood cell (RBC) membrane and making it less flexible and more adherent to endothelial cells.6 SCD shortens the RBC lifespan by having RBCs removed from circulation either by the reticuloendothelial system or through intravascular hemolysis,6 releasing free hemoglobin in the circulation. In addition, RBC sickling and increased cell adhesion lead to endothelial and coagulation cascade activation creating a chronic systemic inflammatory response.7
SCD arises not only from sickle cell anemia (Hb SS), but also from double heterozygosity for Hb S and hemoglobin C in Hb SC disease and sickle β-thalassemia (Hb Sβ), caused by combinations between Hb S and thalassemia mutations that can be either β0 (absent hemoglobin A synthesis) or β+ (with some residual production of normal hemoglobin A).12,6,8 Some heterozygous presentations of SCD may have less severe outcomes,9,10 although such women are still at higher risk of complications, especially during pregnancy and postpartum.
Increased pregnancy and postpartum morbidity2,9,11 and mortality9,11,12 have been recognized in SCD including hypertensive disorders, thromboembolic events, fetal demise, fetal growth restriction, prematurity,13 and maternal death.10,14-16 In addition, SCD complications such as pain crisis, acute chest syndrome, increased susceptibility to infections16,17 and worsening anemia are frequent in pregnancy13 and account for recurrent hospitalizations and mortality. Therefore, SCD is a challenging condition that needs a multidisciplinary antenatal approach.18-20
RBC transfusions are commonly used in SCD to treat severe anemia and to decrease the percentage of circulating Hb S. During pregnancy, they are often utilized on demand but can also be prescribed prophylactically to prevent SCD complications.11
Data on pregnancy outcomes across the different genotypes of SCD vary depending on whether studies were conducted in low- or high-income settings. There is also paucity of data on the placental abnormalities that are expected in a systemic inflammatory condition such as SCD. This study aimed to describe maternal and perinatal outcomes and placental findings across different genotypes of SCD in a tertiary reference center in Southeastern Brazil.
MethodsThis was a retrospective study with medical chart review of SCD cases followed at the Women's Hospital, University of Campinas, Brazil, during antenatal care, childbirth and postpartum. This reference university hospital in Southeastern Brazil provides specialized care to a population of 3100,000. It is located in the same neighborhood as SCD adult and pediatric reference centers.
The study protocol was approved by the local research ethics committee (#14,092,013.4.0000.5404) and individual informed consent was waived since data collection was retrospective with no further interventions. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement checklist was followed in order to standardize the findings considering recommendations for observational studies.21
Data was retrieved from medical records of all the women referred to the institution for antenatal care due to SCD (confirmed by hemoglobin electrophoresis) and that gave birth at the same maternity hospital. Women who gave birth at other institutions were excluded from the final analysis because of lack of information on maternal and perinatal outcomes. For comparison, cases were grouped according to the SCD genotype (Hb SS, Hb SC or Hb Sβ). The variables analyzed included sociodemographic data (maternal age, skin tone, body mass index at first prenatal care visit, previous obstetric history, history of smoking), maternal outcomes and frequency of complications (worsening anemia, pain crisis, acute chest syndrome, occurrence of preeclampsia and maternal near miss), perinatal outcomes (gestational age at delivery, birth weight, 5-minute Apgar score, neonatal complications), placental morphological analysis when available, and any need for blood transfusion. Blood transfusions were classified as prophylactic or on demand according to an institutional protocol established in 2011 that determines the indication for scheduled prophylactic RBC transfusions at around 28 weeks of gestation for SCD cases. Transfusion was considered prophylactic if there was no indication due to an acute complication. Near miss is a condition defined by the World Health Organization (WHO) as a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 42 days of the termination of pregnancy.22 In our study, a near miss was represented by admission to the intensive care unit, RBC transfusion due for acute hemorrhage, eclampsia, or renal or cardiac complications.
Contingency tables were used for categorical variables with statistical analysis using the chi-square test (χ2). The mean, standard deviation, maximum and minimum values were recorded for continuous variables with the Mann Whitney or Kruskal-Wallis tests being used for the comparison of two or three groups, respectively. A p-value <0.05 was considered statistically significant. The EPI-INFO software was used for all analyses.
ResultsBetween January 2011 and May 2017, 67 pregnant women with SCD were referred to the Women's Hospital for antenatal care. Of these, 62 (93 %) gave birth at the same institution with complete data on maternal and perinatal outcomes being available for analysis. According to the genotype, 25 (40 %) women had the homozygous form of the disease (Hb SS) and 37 (60 %) were double heterozygotes with 29 having (47 %) Hb SC and eight (13 %) Hb Sβ (7 - 11 % Hb Sβ0 and 1 - 2 %Hb Sβ+).
All patients had the diagnosis of SCD prior to pregnancy and all of them were followed at a specialized institution. Overall, fourteen women (22 %) had chronic complications due to the disease (such as retinopathy, bone necrosis, stroke sequelae, or pulmonary hypertension), but none of the women were treating acute complications at the moment of pregnancy diagnosis (data not shown).
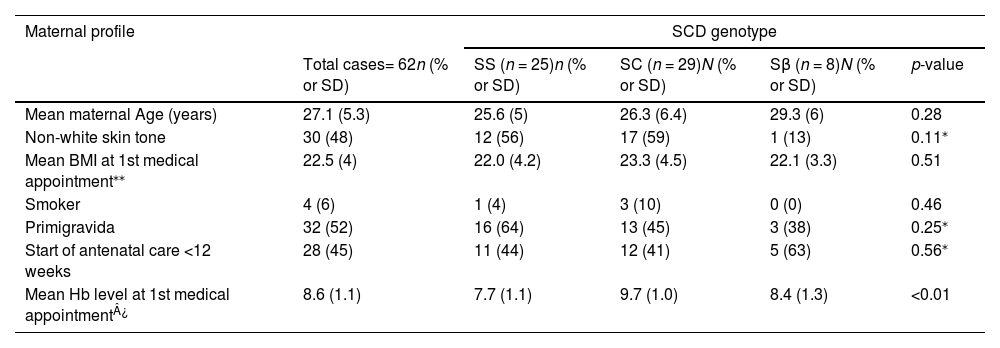
Table 1 shows the sociodemographic and clinical characteristics, and obstetric background of each subgroup. Most of the presented variables did not differ significantly between SCD genotypes. In general, women were of non-white skin tones, it was their first pregnancy and fewer than half of them began antenatal care within the 1st trimester. The mean maternal age was similar in the three groups with an average age of 27.1 years. The only significant difference was in hemoglobin level at the first antenatal care visit, which was significantly lower in the homozygous genotype (7.7 g/dL) compared to Hb SC and Hb Sβ (9.7 g/dL and 8.4 g/dL, respectively; p-value = 0.01).
Sociodemographic and clinical characteristics, and obstetric background of Sickle Cell Disease subgroups (Hb SS, Hb SC and Hb Sβ).
| Maternal profile | SCD genotype | ||||
|---|---|---|---|---|---|
| Total cases= 62n (% or SD) | SS (n = 25)n (% or SD) | SC (n = 29)N (% or SD) | Sβ (n = 8)N (% or SD) | p-value | |
| Mean maternal Age (years) | 27.1 (5.3) | 25.6 (5) | 26.3 (6.4) | 29.3 (6) | 0.28 |
| Non-white skin tone | 30 (48) | 12 (56) | 17 (59) | 1 (13) | 0.11⁎ |
| Mean BMI at 1st medical appointment⁎⁎ | 22.5 (4) | 22.0 (4.2) | 23.3 (4.5) | 22.1 (3.3) | 0.51 |
| Smoker | 4 (6) | 1 (4) | 3 (10) | 0 (0) | 0.46 |
| Primigravida | 32 (52) | 16 (64) | 13 (45) | 3 (38) | 0.25⁎ |
| Start of antenatal care <12 weeks | 28 (45) | 11 (44) | 12 (41) | 5 (63) | 0.56⁎ |
| Mean Hb level at 1st medical appointment¿ | 8.6 (1.1) | 7.7 (1.1) | 9.7 (1.0) | 8.4 (1.3) | <0.01 |
SD: standard deviation; BMI: body mass index; Hb: hemoglobin.
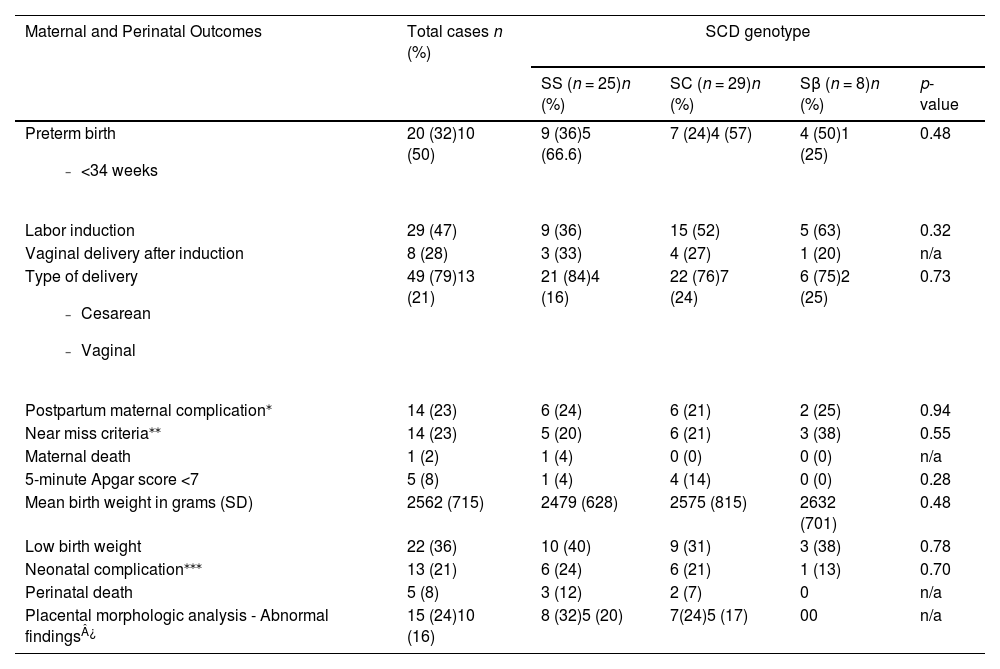
Maternal and perinatal outcomes are shown in Table 2. Prematurity was high in all the genotypes (32 % in Hb SS, 28 % in Hb SC, and 50 % in the Hb Sβ group; p-value = 0.48). Induction of labor was used in 36 % (n = 9) of the Hb SS women, 52 % of the Hb SC women (n = 15) and 63 % of the Hb Sβ women (n = 5), with success rates of vaginal delivery of 33 %, 27 % and 25 %, respectively. Cesarean section was the route of delivery in 79 % of all cases, with 84 % for the homozygous form of the disease. Among women undergoing labor induction, 72 % had a Cesarean section (66 % in the Hb SS group - 4 failed induction, 2 due to fetal distress, 73 % in the Hb SC - 7 failed induction, 2 due to fetal distress and 2 due to maternal sickle cell complications during labor, and 80 % in the Hb Sβ group - 3 failed induction and 1 maternal sickle cell complication during labor).
Maternal and Perinatal Outcomes of Sickle Cell Disease patients according to genotype.
| Maternal and Perinatal Outcomes | Total cases n (%) | SCD genotype | |||
|---|---|---|---|---|---|
| SS (n = 25)n (%) | SC (n = 29)n (%) | Sβ (n = 8)n (%) | p-value | ||
Preterm birth
| 20 (32)10 (50) | 9 (36)5 (66.6) | 7 (24)4 (57) | 4 (50)1 (25) | 0.48 |
| Labor induction | 29 (47) | 9 (36) | 15 (52) | 5 (63) | 0.32 |
| Vaginal delivery after induction | 8 (28) | 3 (33) | 4 (27) | 1 (20) | n/a |
Type of delivery
| 49 (79)13 (21) | 21 (84)4 (16) | 22 (76)7 (24) | 6 (75)2 (25) | 0.73 |
| Postpartum maternal complication⁎ | 14 (23) | 6 (24) | 6 (21) | 2 (25) | 0.94 |
| Near miss criteria⁎⁎ | 14 (23) | 5 (20) | 6 (21) | 3 (38) | 0.55 |
| Maternal death | 1 (2) | 1 (4) | 0 (0) | 0 (0) | n/a |
| 5-minute Apgar score <7 | 5 (8) | 1 (4) | 4 (14) | 0 (0) | 0.28 |
| Mean birth weight in grams (SD) | 2562 (715) | 2479 (628) | 2575 (815) | 2632 (701) | 0.48 |
| Low birth weight | 22 (36) | 10 (40) | 9 (31) | 3 (38) | 0.78 |
| Neonatal complication⁎⁎⁎ | 13 (21) | 6 (24) | 6 (21) | 1 (13) | 0.70 |
| Perinatal death | 5 (8) | 3 (12) | 2 (7) | 0 | n/a |
| Placental morphologic analysis - Abnormal findings¿ | 15 (24)10 (16) | 8 (32)5 (20) | 7(24)5 (17) | 00 | n/a |
SCD: sickle cell disease; n: number of patients; SD: standard deviation.
Admission to intensive care unit, red blood cell transfusion due to acute hemorrhage, eclampsia, renal or cardiac complications.
Near miss was a condition found in 23 % of cases in all three genotypes. Low birth weight was present in 40 % of the Hb SS group, 31 % of the Hb SC group and 38 % of the Hb Sβ group. Neonatal death (2 cases) occurred only in the homozygous group.
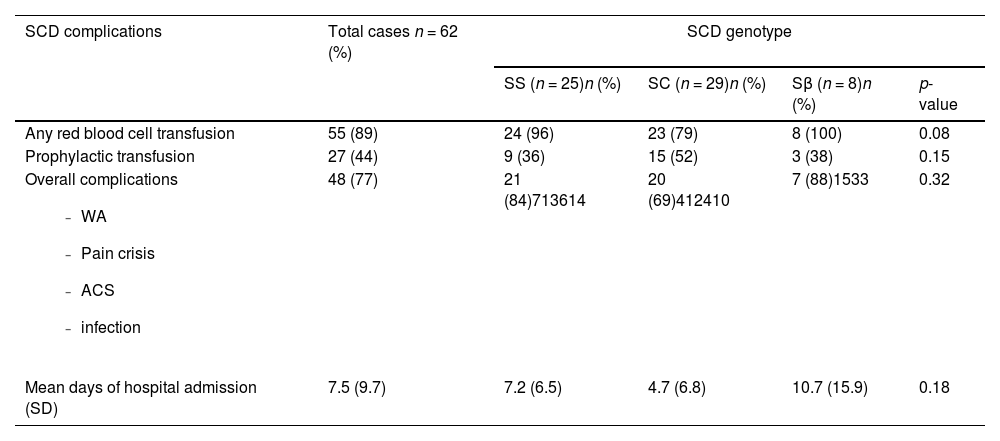
The SCD-related complications are described in Table 3. Almost all women needed at least one RBC transfusion during pregnancy (96 % with Hb SS, 79.31 % with Hb SC and 100 % with Hb Sβ); the trend was towards less transfusions in Hb SC women (p-value = 0.08). Prophylactic blood transfusions were provided for 38 %, 65 % and 38 % of the Hb SS, Hb SC and Hb Sβ groups, respectively (p-value = 0.15). SCD complications occurred in 84 % of Hb SS, 69 % of Hb SC and 88 % of Hb Sβ women (p-value = 0.32). These complications included worsening anemia, pain crisis, acute chest syndrome and infections. Preeclampsia occurred in 15 % of women and was not statistically different across genotypes. One maternal death occurred in the Hb SS group, a non-white woman with late strat of prenatal care, who was hospitalized due to a pulmonary infection at 29 weeks gestation that evolved to septicemia and refractory shock (Figure 1).
Sickle cell disease complications among pregnant women during antenatal care, childbirth, and postpartum.
SCD: sickle cell disease; WA: worsening anemia; ACS: acute chest syndrome; SD: standard deviation.
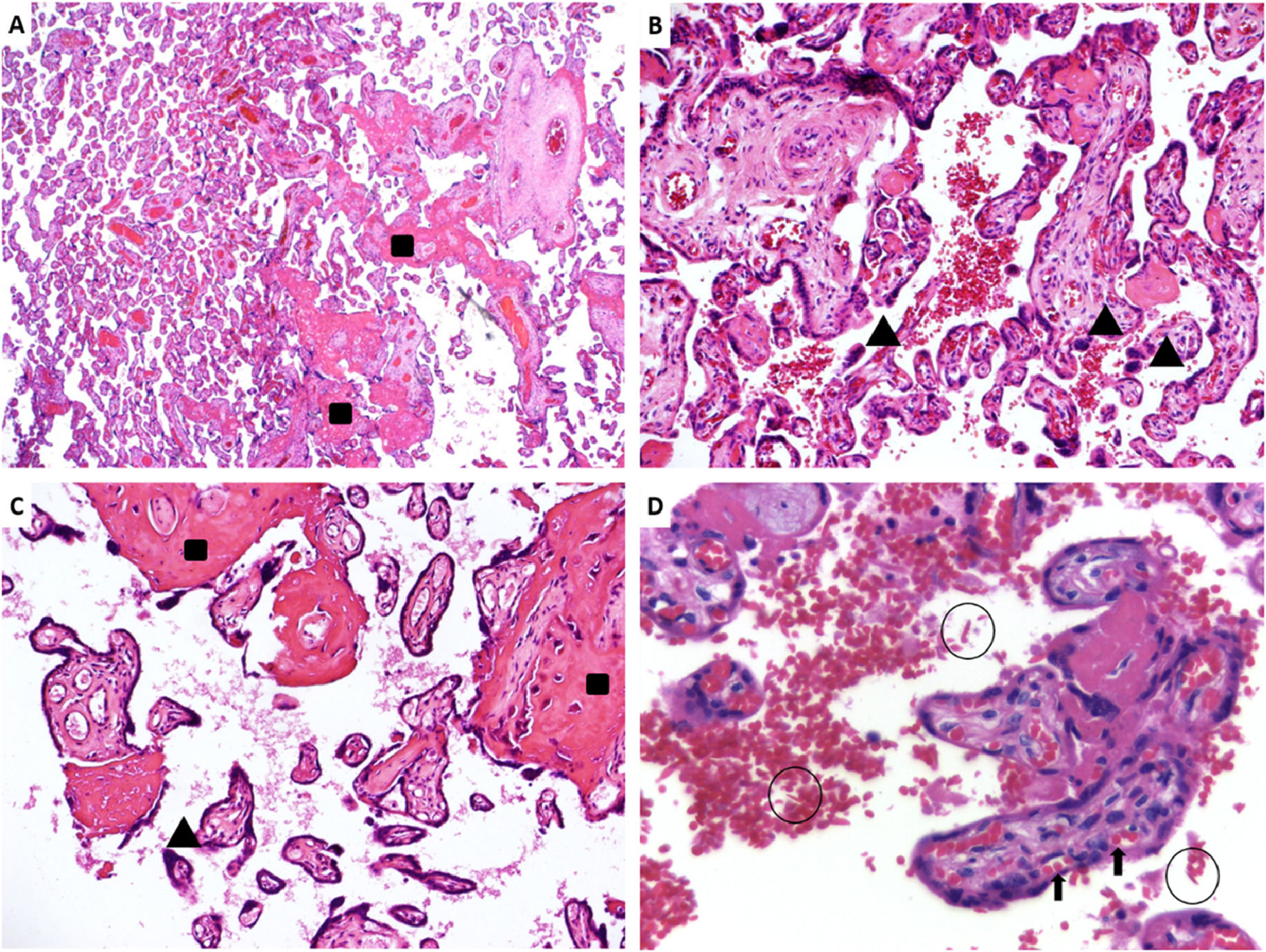
Placental pathology was available in 15 cases (8 Hb SS and 7 Hb SC), with abnormal findings in ten (5 Hb SS and 5 Hb SC). From the 10 placentas with abnormal findings, five placentas were hypoplastic (defined as weight below the 10th centile for gestational age), two of them severely affected (below the 3rd centile), nine showed microscopic features of maternal vascular malperfusion (MVM), formerly known as utero-placental hypoxia. MVM can be divided in two ways: global/partial and segmental/complete.23 Half of the placentas showed global/partial MVM, with small or short hypermature villi for gestational age, accompanied by an increase in syncytial knots, characterizing accelerated villous maturation. Moreover, four placentas presented infarctions, characterizing segmental/complete MVM. Two of the nine placentas showed both patterns of MVM. Extensive infarcts were considered the cause of stillbirths that occurred in two Hb SC patients. Only one patient showed decidual arteriopathy. Five placentas showed accentuated perivillous fibrin deposition (1 Hb SS and 4 Hb SC), and ten cases had sickled erythrocytes in the intervillous space (7 Hb SS and 3 Hb SC). Figure 2 shows representative images of the placental findings.
Sample images of placental histopathology in sickle cell disease patients. A. A panoramic view of a pre-term sickle cell disease placenta with mature term villi. HE: 25x. B. Hypermature villi with syncytial knots. HE: 100x. C. Hypermature villi, with increased syncytial knots and accentuated inter- and perivillous fibrin deposition. HE: 100x. D. Many sickled red blood cells within the intervillous space (circles); normal fetal red blood cells in the villous vessels (arrow). HE: 400x. Rectangles (increased fibrinoid deposition), arrowheads (syncytial knots), sickled red blood cells (circles), fetal red blood cells (arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Regardless of genotype, SCD is a condition associated with an increased incidence of adverse maternal and perinatal outcomes, with high frequencies of neonatal morbidity, preterm birth, need of cesarean section and maternal near miss. In this study, Hb SS presented the lowest baseline hemoglobin level and the only case of maternal death in the analysis.
Hb SS is usually the most frequent genotype of SCD patients, followed by Hb SC and then by Hb Sβ, but this can vary according to the country and region.24 In this retrospective study of pregnancies in women with SCD, Hb SC was the most frequent genotype, corresponding to almost half of the cases (47 %); this is different to previous Brazilian reports in which Hb SS was predominant.11,25,26 The Hb Sβ genotype accounted for the least number of cases and were mostly the Hb Sβ0 subtype (7/8 patients). Hb Sβ0 is the most severe form of Hb Sβ because it also causes the production of over 90 % of Hb S, similar to what is found in Hb SS; both conditions are clinically indistinguishable. The majority of Hb SS and Hb SC cases had non-white skin tones, in agreement with previous studies, as SCD is more common in African-descendants.27 The relatively high prevalence of European-descendants in the current Hb Sβ group can be explained by admixture in the Brazilian population with the co-inheritance of β-thalassemia mutations of predominantly Portuguese and Italian origins.28
It is important to notice that less than 50 % of SCD women started antenatal care during the first trimester. We believe that unplanned gestation and delays in referral to obstetric care may have played a role in contributing to this. SCD is a condition associated with high morbidity during pregnancy, therefore, a planned gestation, with early scheduled antenatal care would be key towards more adequate healthcare.29,30
The mean maternal age in this study was 27 years and almost half of the women were multiparous. The recurrence of gestation in this population with high risk of complications may highlight gaps in family planning programs. A previous Brazilian study about reproductive issues in SCD did not find differences in the age at first pregnancy (mean 28 years), number of pregnancies or number of living children between the three SCD genotypes.31 There are two other similar Brazilian studies - also from settings in the southeast of the country (state of São Paulo) - one that analyzed 55 cases from São Paulo32 and the other with 34 cases from Ribeirão Preto.18 Both had comparable mean maternal ages: 26.7 and 23.9 years, respectively
Prematurity, mostly due to medical indication for preterm delivery, is a common adverse outcome in SCD patients. The worldwide prematurity rate is 11.1 % according to the WHO33 with Brazilian national data estimating an incidence from 9.9 to 12.3 %.29 SCD rates of prematurity vary from 4.1 % in one study with 37 women in Turkey34 to 50.4 % in a study with 109 women in the United Kingdom.9,10 The present study found high rates of prematurity, with an overall incidence of 32 %, due to either a maternal condition or fetal distress. The Brazilian study from Ribeirão Preto had a prematurity rate of 23.5 %18 and the one from São Paulo 34.5 %,32 both in agreement with the literature and the findings of the current analysis. Half of preterm deliveries in this study were before 34 weeks of gestation and were related to more severe adverse outcomes among the neonates. More than one third of the newborns in this study had low birth weight (less than 2500 g) and 8 % presented a 5-minute Apgar score below 7, both conditions are associated with adverse short- and long-term outcomes. We found no significant differences in prematurity between genotypes, while a study from the UK found a higher prematurity rate in Hb SS than in Hb SC (47.1 % versus 20.5 %; p-value = 0.01).9 This discrepancy may be due to the smaller number of cases in the present study, which analyzed women from a single tertiary hospital but it suggests that providers should not rely on genotype to predict chances of prematurity.
Labor induction was attempted in almost half of the cases of these patients, in more than a third of the Hb SS and more than a half of the Hb SC and Hb Sβ women. This figure is higher than another study that compared Hb SS to Hb SC women, with an overall induction of labor rate of 39.44 %. In both studies the difference between the genotypes was not significant.10 During labor induction and labor itself, it is important to monitor hydration, symptoms of possible complications and methods for pain relief, including early analgesia. Evaluating the need of blood transfusion prior to induction is also recommended as blood banks may have difficulty finding compatible blood for alloimmunized patients.35
A high rate of cesarean sections was observed. A review of literature describes rates from 16 to 91 %.36 One study from UK found an overall prevalence of 37.61 % with 52.9 % related to the homozygous genotype.10 Nevertheless, Brazil is among the countries with the one of the highest cesarean delivery rates in the world.37 A Brazilian study from 2022 in São Paulo reported a 82.7 % cesarean section rate32 while a study conducted in Ribeirão Preto in 2014 reported a Cesarean section rate of 41.1 %.18 Therefore, the results of this study should not be interpreted as evidence that SCD leads to the need for cesarean sections more often than other comorbidities during pregnancy, nor does it mean that SCD is an indication for cesarean section.
Comparing the present findings to another study in the same institution with a case series of seven patients from 2002 to 2004, there was an increase in the number of pregnancies (7 pregnancies in three years versus 67 pregnancies in six years) and an increase in pregnancies among patients with the Hb SC genotype. We also found the persistence of a high prevalence of complications and cesarean sections showing that, despite a time interval of almost two decades with better access to treatment and safe transfusions, the frequency of complications remains high. The number of pregnancies increased almost ten-fold, possibly due to more patients surviving to childbearing age due to more effective treatment and follow-up of SCD during childhood and adolescence, thereby preserving fertility.38
Nevertheless, SCD is a condition that affects women in all socioeconomic classes and in high- and low-income countries. The disease is an important risk factor for hypertensive disorders, fetal growth restriction, infections, thromboembolic events and maternal death.39-41 Our overall preeclampsia rate was 15 %, with no difference between the genotypes. This rate is higher than that reported for a population without hemoglobin disorders, but similar to that described in a study in the US.42 It is also lower than another Brazilian study conducted in Northeastern Brazil which found higher rates of preeclampsia in all three genotypes (23 %)26 and higher than the other two studies conducted in the same Brazilian southeastern region (10 %18 and 5.4 %32). The lower frequency of preeclampsia in the current study may be due to a local institutional protocol recommending the prophylactic use of calcium and acetylsalicylic acid for all SCD women as they are known to be at higher risk for hypertensive complications. There is also a recommendation in our institution to consider the use of prophylactic RBC transfusions at around 28 weeks of gestation. Further studies are needed to define the real individual impact of each intervention. A meta-analysis in 2015 found more than a two-fold risk for preeclampsia in SCD patients and a five-fold risk for eclampsia in Hb SS women.9 A retrospective cohort with 344 women with SCD demonstrated a four-fold risk for preeclampsia with severe features among those with SCD.41
The current data are in accordance with studies worldwide describing overall maternal morbidity mainly due to pain crises, acute chest syndrome, infections and severe anemia with a need for RBC transfusions.2,9 Maternal complications occurred in the majority of women, with almost 90 % receiving RBC transfusions, of which only 44 % were indicated prophylactically. There was a trend towards a difference between groups in respect to the need for transfusions, suggesting that Hb SC patients required less transfusion, which is expected since baseline anemia was also less severe in Hb SC women than in others. Chronic anemia is a characteristic of the disease and RBC transfusions are frequently necessary for these patients. There is lack of evidence to recommend specific hemoglobin thresholds during pregnancy, so monitoring for fetal complications, frequency of maternal complications and prior history of a complicated pregnancy should be considered in the physician's decision to transfuse on demand or prophylactically.
In 2016, a meta-analysis described the frequency of pain crises ranging from 0.4 % to 77.8 % and the frequency of pulmonary complications (including acute chest syndrome), one of the main causes of maternal death,43 ranging from 0.4 to 29.6 %.36 In this study, acute chest syndrome complicated 19 % of pregnancies and pain crises around half of the cases. A Brazilian study from Ribeirao Preto reported a rate of acute chest syndrome of 29 %,18 while a study from São Paulo city reported 12.7 % cases, all with the Hb SS genotype.32
The maternal near-miss rate was 23 %, meaning that one woman in five almost died in pregnancy and/or postpartum, thereby stressing the severity of the disease and the importance of referral to a high-risk healthcare facility to treat these women. The WHO defines maternal near miss as a condition in which a woman nearly died, but survived a complication that occurred during pregnancy, childbirth or within 42 days after the termination of pregnancy. Women survive by chance or due to precise medical interventions. They have many similar characteristics to those that die. Near miss is an important health indicator that enables the identification of weaknesses in obstetric care.22 We reported one maternal death in the Hb SS group (overall rate of 2 % among all SCD women), similar to the Brazilian study with 34 patients that also reported one death of a Hb SS woman in the postpartum period due to acute chest syndrome.18 These data are in contrast with past decades when maternal mortality rate was around 11 %.44 This can be attributed to an improvement in maternal care and surveillance with a better understanding of the clinical presentation of SCD-related complications and life-threatening situations.
While we had a limited number of placental specimens analyzed for morphologic abnormalities, two-thirds of the cases were found to have placental hypoplasia and infarcts, which may be caused by tissue ischemia due to SCD vaso-occlusion in the placenta, and may also explain the occurrence of fetal growth disorders and poor perinatal outcomes commonly regarded as secondary to ‘placental insufficiency’.45 Such abnormalities were found regardless of SCD genotype, suggesting once again that genotype is a poor predictor of complications during pregnancy. Considering that our patients were already broadly treated with transfusions and acetylsalicylic acid, and that currently available treatments for SCD are contraindicated during pregnancy,46 other interventions to treat SCD during pregnancy need to be developed. Further larger studies are warranted to investigate risk factors for placental abnormalities in SCD as well as to define pathophysiological mechanisms of these abnormalities to propose novel effective treatment modalities and prevention strategies.
This study has limitations: it was retrospective, based on medical records that are not always standardized. The number of cases was limited as data were collected in only one tertiary medical service and a few patients did not give birth at the same institution. On the other hand, an important strength of this study is the setting: being the only tertiary center providing specialized care for SCD patients, we were able to capture over 90 % of all SCD pregnancies in the metropolitan area during a period of seven years. We were also able to compare outcomes across the three main SCD genotypes and reviewed placental findings which have not been reported previously. Finally, the same institutional protocols to treat SCD during pregnancy were applied to all patients, reducing the effects of heterogeneous care when comparing different genotypes over time.
In summary, we demonstrated that SCD remains a disease with high maternal and perinatal morbidity, with high rates of complications in all SCD genotypes and with frequent placental structure abnormalities. Larger prospective studies are needed to identify risk factors for complications other than genotype and to develop new strategies to improve outcomes. Also, larger multicentric and multidisciplinary studies can help improve the use of prophylactic RBC transfusions, which could be indicated earlier in pregnancy. Finally, there is a lack of high quality evidence to optimize the use of fetal scans during antenatal care to identify early complications and reduce prematurity and its consequences in SCD pregnancies.