Organizing Pneumonia (OP) is classified as an Idiopathic Interstitial Pneumonia (IIP).1 Clinically, the patients present dyspnea, cough, and low fever, and usually respond well to corticosteroids, reaching clinical and radiologic resolution in a short timeframe. However, sometimes infiltrates do recur and may not resolve. Radiologically, migratory consolidations are identified in high-resolution computed tomography (HRCT), sometimes associated with progressive fibrosis with reticulation and areas of persistent consolidation.1 The histology reveals “proliferation of fibroblastic tissue within small airways, alveolar ducts, and alveolar spaces”.2 OP can be secondary to other pathologies such as common variable immunodeficiency3 and malignant disease among others.4 At times no etiology is identified and the term Cryptogenic Organizing Pneumonia (COP) is used.1 Furthermore, OP could coexist or mimic a malignant disease.4,5
We present a case of a 56-year-old (y/o) man, ex-smoker of 19 pack-years, an engineer without any contact with chemical products. He lived in a flat without detectable exposures, and indicated no alcohol or drug abuse. In 2002 (41 y/o), he was monitored by other center due to dispnea, non-productive cough and low-grade fever. There were no remarkable indicators during physical examination (no pathological breathing sounds, 98% oxygen saturation at room air). Laboratory studies highlighted neutrophil-predominant leukocytosis and elevated level of C-reactive protein. X-ray findings and High-Resolution Computed Tomography (HRCT) showed lung infiltrates that migrated from one relapse to the other. We do not dispose from other data (bronchoalveolar lavage…). Initially, the clinical situation and infiltrates had complete remission with 15mg per day of corticosteroids (CS). He remained asymptomatic for a long period of time (several months to a year). However, the time he remained free of infiltrates shortened and required increasing doses of cortisone up to 30mg per day. Subsequently the patient could not discontinue corticosteroid use because his respiratory condition worsened during the periods free of infiltrates. He became CS-resistant in 2011 requiring 15mg per day minimum. During this time, he required hospitalization for some of the recurrences (2002, 2004(x2), 2008, 2011). For every hospitalization, he had migrating chest infiltrates and neutrophilic leukocytosis without any evidence of infection. An open surgical lung biopsy was performed by videothoracoscopy (VATS) in order to confirm a diagnosis in April 2013 under 25mg/day of prednisone. Anatomic pathological findings were described as septal thickening and paraseptal fibrosis without fibroblast foci (FF). The case was then referred to our center, under prescription of CS and azathioprine. Azathioprine was quickly removed because of gastric intolerance.
During the first visit at our center in August of 2013, a new infiltrate at the upper right lobe with mediastinal lymph nodes was detected and it increased progressively through one year of follow-up. Our anatomopathologist (Dr. Llatjos) reviewed the previous lung biopsy and agreed with the clinical description of unspecific fibrosis without FF, however no specific diagnosis could be done. Patient declined another surgical lung biopsy or cryobiopsy. A Bronchoalveolar Lavage (BAL), CT-Guided Tru-Cut (Fig. 1A and B) and an EBUS were performed. BAL showed a dominance of macrophages. Lung and adenopathy cytologies showed lymphocytic infiltrates without, atypia nor lymphoma markers and with negative cultures. The patient required high doses of CS (prednisone 40mg per day) at this time. In order to diminish the side effects of prednisone, mycophenolate mofetil (MMF) was initiated but it was discontinued after 6 months due to no clinical improvement. Several laboratory studies (proteinogram, lymphocyte subsets, hormones, autoimmunity, antibodies, and precipitins, among others) and urine analyses (ions, proteins and globulins excretion) were performed searching for other causes. Only hypogammaglobulinemia was detected (Immunoglobulin G 438mg/dL and Immunoglobulin A 41mg/dL) without markers of hematological, oncological or autoimmune disease. Then immunoglobulin replacement therapy3 was dispensed for a year, but was ceased due to lack of clinical improvement. Pulmonary function tests reflected a progressive restrictive pattern of decreasing forced vital capacity (FVC) from 2013 to 2016 (from 3,330L [77% of the predicted value] to 2,150L [56,7% of the predicted value]) and a gradual decrease in diffusing lung capacity for carbon monoxide (DLCO) from 71% to 56.1%.
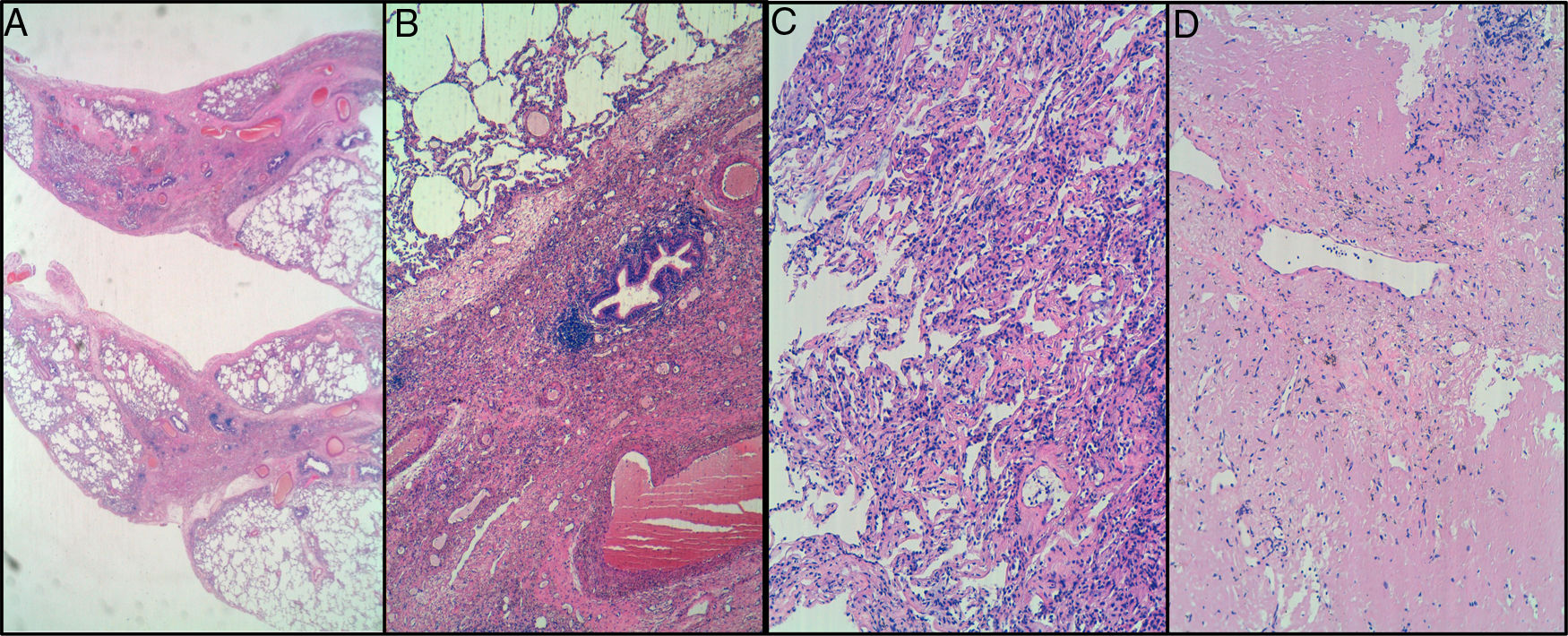
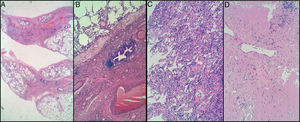
CT-Guided Tru-Cut. A) interstitial fibrosis with mainly peribronchiolar distribution, but no fibroblastic foci observed. B) mild interstitial chronic inflammation. Lymphocytic infiltrates without atypia. No intraluminal organizing fibrosis in these samples. Cryobiopsy in right upper lobe. C) mild interstitial chronic inflammation. No atypical cells suspicious for malignancy were observed, either lymphoid or epithelial. D) pulmonary fibrosis with complete remodeling of lung architecture in one of three fragments. Elastosis and amyloidosis were ruled out.
In February of 2016, a new infiltrate in the right basal lobe appeared with enlargement of lymph nodes. Cryobiopsies (performed in August, September and November of 2016) (Fig. 1C and D) from the upper and basal right lobes revealed collagen, elastin and lymphocytes without cellular atypia. Another EBUS was performed (January of 2017) and again revealed no cellular atypia or lymphoma markers. Laboratory analysis showed elevation of Plasmatic β2-microglobulin, plasmatic lambda chains and urine monoclonal kappa light chains. Bone marrow and subcutaneous fat aspiration were performed without finding any structural, cytological or haematological alteration. Then, biopsies (open lung biopsy (2013) and criobiopsies (2016)) were re-evaluated by another expert anathomopathologist from the Instituto Nacional de Enfermedades Respiratorias (INER, México; Dr. Gaxiola) who coincide with the description of our anathomopathologist and could not give a specific diagnosis either. Furthermore, the case was exposed to the CRAMPID goup (Clinical-Radiological-Anathomopathological ILD group from Catalunya) and expert ILD-radiologists defended fibrosing OP as the radiologic diagnose (Dr. Franquet, Dr. Luburich). The patient needed several hospitalizations because of respiratory insufficiency requiring elevated doses of corticosteroids. A PET/CT was performed at the end of 2017, exhibiting hypermetabolical lesions in lung, mediastinal lymph nodes and a nodular centimetric lesion adhered to psoas.
Biopsy of the psoas lesion was compatible with a high-grade diffuse large B-cell lymphoma (DLBCL). Rituximab was initiated in monotheraphy due to comorbidities and Eastern Cooperative Oncology Group (ECOG) status. After the third dose, the patient died due to a Pseudomonas sepsis caused by severe neutropenia. Necropsy revealed DLBCL (Fig. 2A–D) affecting the retroperitoneum and right lung, with adjacent OP and diffuse alveolar damage. Classical Hodgkin lymphoma (HL) was evidenced in the mediastinal lymph nodes (Fig. 2E–H).
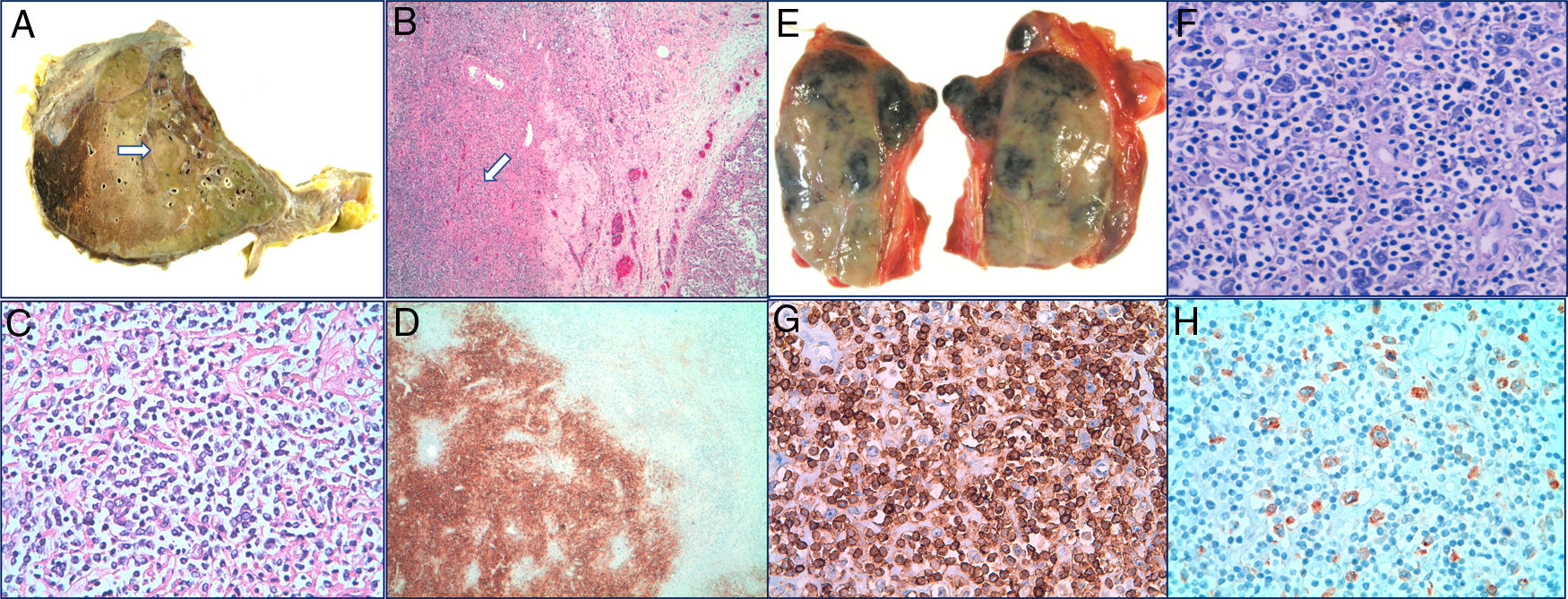
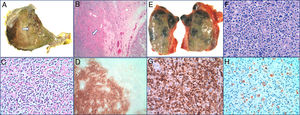
Autopsy: Right lung with Diffuse Large B-Cell Lymphoma (DLBCL). A transverse section of the right lung showing a 3.8cm solid and cream colored nodule (arrow). B Lung parenchyma with presence of DLBCL (arrow) (H-E 40×). C Proliferation of large lymphocytes (H-E 400×). D Immunohistochemical stained for CD20 that demonstrates the B lineage of proliferation (40×).
Mediastinal lymph node with Classic Hodgkin Lymphoma. E Mediastinal adenopathy of 5.5cm. F Hodgkin cells in a cellular background rich in lymphocytes and containing histiocytes (H-E 400×). G Immunohistochemical staining for CD3 (200×). H Immunohistochemical staining for CD30 (200×).
In this case, two different forms of aggressive lymphoma (DLBCL and HL) coexisted in the necropsy, which is very unusual.6–8 Although there had been extensive investigations, the appearance of malignancy 4 years before death could not be proven. The diagnosis of lymphoma simultaneously with OP has already been described4,5; however, there is no evidence of association of OP before aggressive lymphoma detection, and in this case there is not evidence of hematologic malignant transformation.6–8 This case never demonstrated histological evidence of OP alive (only in the necropsy). This opens the case to the discussion of several questions.
The first question is whether the lymphoma could have been present in the lung for years, but modulated by the continuous steroid use. The diagnosis of the lung lymphoma was Hodgkin’s lymphoma, which didn’t respond to years of steroid use. Only low-grade lymphomas (marginal zone, small lymphocytic lymphomas) respond to steroids, and only for a short period of time.
Another issue to consider is whether the lymphomas could have resulted from continuous immunosuppression. Hodgkin’s lymphoma and EVB negative DLBL are not usually associated with immunosuppression. The classical lymphomas associated with immunosuppressive treatment9/solid organ transplant10 are usually EVB-related with an aggressive clinical presentation if they are not treated. However, EBV was negative in our patient and he received only 6 months of MMF at the time when the infiltrate was already persistent.
An additional question is whether a repeated open chest biopsy, refused by the patient, could have led to an earlier lymphoma diagnosis. Unfortunately, the patient rejected it several times, as he had already undergone multiple lung biopsies (cryobiopsy and EBUS) in the endeavor to diagnose this young patient.
Another inquiry is whether all sites of increased metabolism in PET, including mediastinal nodes and lung, should have been explored. Usually, all sites PET positive in lymphoma patients are not biopsied assuming that all sites are going to be the same lymphoma subtype. In this patient, psoas biopsy was more accessible than the other localizations, and gave the diagnosis of DLBCL. The other sites we considered the same histology, and therefore unexpected in the necropsy to find Hodgkin’s lymphoma in the lung.
The last question is to determine whether rituximab, as the only treatment, was the right choice at that juncture for the treatment of DLBCL. When the diagnosis was made (only Diffuse large B cell lymphoma, Hodgkin’s lymphomas diagnosis was elucidated in the necropsy), the patient had a poor ECOG with an active infection, so the hematology department decided to administer only Rituximab to avoid neutropenia, a spread of the infection.
Meeting of ethical standards
We declare that we follow all of ethical standards of Elviser group.
We declare that anonymity of the patient has been take care and there isn’t identifiable characteristic or identifiable-images of the patient.
This article isn’t a clinical trial.
Conflict of interest notificationThe authors have no commercial or financial interests related to this study to disclose.