“Apheresis” is a Greek word which means “withdrawal” or “removal”. Thus, “plateletpheresis” refers to a procedure in which a portion of platelet components is removed, along with a small amount of plasma, and the remaining components are returned to the blood donor. The product is also known as single donor platelet (SDP). In the Cobe Spectra procedure, the component was named extended life platelet (ELP).
The COBE Spectra (Caridian BCT, Lakewood, CO) is one of the apheresis machines by which plateletpheresis can be performed, along with simultaneous leukoreduction. It is a highly automated, microprocessor-controlled instrument1 and has preloaded programs for several procedures, but some manual control is also possible. An anticoagulant is pumped through the inlet line in a controlled anticoagulant (AC)/whole blood (WB) ratio by a dedicated anticoagulant pump. A Leukoreduction System (LRS) was introduced into the Spectra in version 5.1 to minimize the leukocyte content of the platelet components. This LRS separates the white blood cells from platelets using the principle of elutriation, based on the size and density of cellular components in the blood. The LRS includes a modified dual-stage channel and modified computer software to regulate and monitor the pumps and sensors.
A unique feature of this instrument is the online collection concentration monitor (CCM). During plateletpheresis, this device monitors for leukocyte and red cell spillovers and estimates optically how many platelets have been collected. If a spillover occurs, the CCM re-positions the collect valve to return the resulting contaminated product to the donor until the line has cleared, as determined by the operator. With the LRS system, the CCM is made more sensitive. Platelet collection was enhanced with software Version 7.0 (LRS Turbo) by modifying the dual stage channel and the computer programs to process blood faster during the establishment of the interface.2
The conical LRS chamber enhances the separation of platelets from leukocytes with saturated, fluidized, particle bed filtration technology called elutriation. The collection line contents enter the LRS chamber at a high speed, owing to the small tubing diameter, but slow down as they encounter the increased diameter of the chamber. Leukocytes tend to stay at the chamber entrance while platelets move radially inward. When the chamber has filled with platelets, they begin to exit. Two forces tend to keep leukocytes at the chamber entrance: (1) a higher g force in the outer portion of the chamber and (2) the mass of less dense platelets they would have to percolate through to exit the chamber. The system can consistently collect platelet components with <1.0 × 106 leukocytes.3
Various troubles may be faced during plateletpheresis procedure.4 The RBC contamination (spillage) is managed by changing the donor input, such as increasing the donor hematocrit value by 3 points. In cases where there was no increment in the CCM, a maneuver, such as increasing the collect pump flow rate and decreasing the plasma pump rate, may also help and the hematocrit value of the donor can be reduced if the interface had not been formed properly, as visualized from the centrifuge door of the Cobe Spectra. We hereby report a unique trouble-shooting in which there was no increment in the CCM after 45 min of procedure and the LRS chamber had trapped all the platelets separated during plateletpheresis procedure.
Case reportA 25-year-old healthy male blood donor presented to our department for platelet donation. Donor screening was performed as per criteria established by the Drugs and Cosmetic Act.5 The venous status of both arm cubital veins was observed. Height and weight were recorded and blood samples were obtained for complete blood count, confirmation of blood group and screening for transfusion-transmitted infections. The donor hemogram showed hemoglobin of 163 g/l, total leukocyte count of 5.3 × 109/l and platelet count of 206 × 109/l. The donor was found eligible for the plateletpheresis procedure. Written consent was obtained before commencement of the procedure.
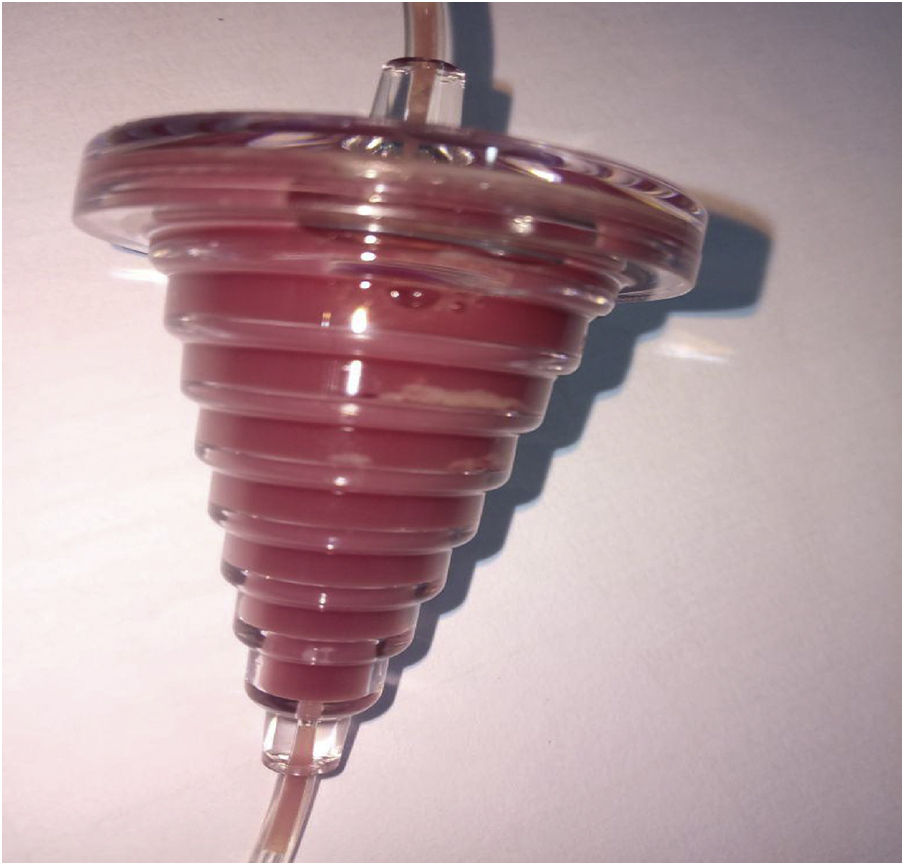
The ELP collection was started using the Cobe Spectra cell separator. The procedure ran smoothly from the beginning and there was no alarm. At 14 min of procedure, the collect valve switched automatically to the open position. However, after 25 min of procedure, the platelet yield in the collect concentration monitor was only 0.1, and this is not usual. Furthermore, normal swirling of platelets was not observed in the collection bag. All donor input data were checked and found to be accurate. There was no increment in the CCM platelet yield for the next 5 min. Higher donor hematocrit input is a known reason for the non-increment of CCM, so the hematocrit was reduced by 3 points and the procedure was observed for the next 15 min.6 During this period, only plasma was being collected in the platelet collection bags and there was no swirling inside them. Meanwhile, the manufacturer’s technical expert was informed. After 60 min of procedure, the CCM yield was 0.2 and there were no swirling visible by the naked eye inside the platelet collection bag. As per instruction by the technical specialist, we stopped the spin and the centrifuge chamber was opened. There was no leakage or kink in the tubing inside. Everything was in place and nothing was abnormal, except the LRS chamber, which appeared to be filled with white blood cells, and probably it had trapped all the platelets inside, as shown in Figure 1. As there was no swirling in the platelet bag, it was high likely that the LRS chamber, which is supposed to trap only leukocytes, had trapped all the platelets. At this time, we tapped the LRS chamber a few times manually so that trapped platelets would be released. The centrifuge door was closed and the procedure was resumed. This maneuver worked to some extent and the CCM raised from 0.2 to 0.5 in the next 7 min. The platelets released from the LRS chamber now started collecting. However, after 7 min, a spillover alarm sounded, indicating that the collection tube had some vacuum or air inside. Meanwhile, we observed that the procedure was about to be completed and that 284 ml of anticoagulant had already been delivered to the donor. As the donor also appeared to be a little apprehensive, the procedure was aborted after 72 min, considering the donor safety and meager product yield. The rinse back was completed at 76 min of procedure.
Laboratory parametersDonor platelet depletion was evident from the pre- and post-procedure platelet count in the hematology analyser, from the initial total platelet count of 206 × 109/l to 128 × 109/l post-procedure, as shown in Table 1. Subsequently, the platelet count from the LRS chamber of 1310 × 109/l in a 1:10 dilution confirmed the trapping of platelets as the reason for the failure in their collection.
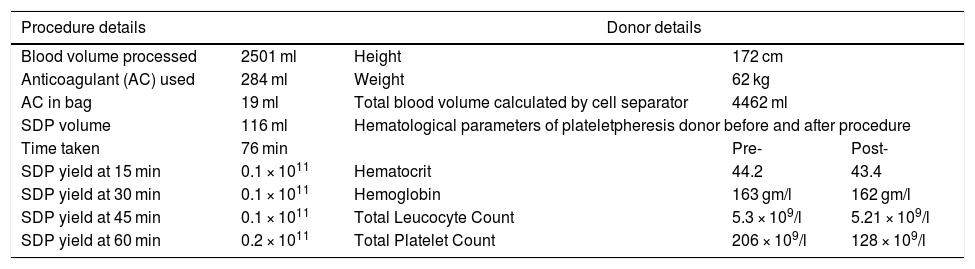
Plateletpheresis donor details with procedural parameters.
| Procedure details | Donor details | |||
|---|---|---|---|---|
| Blood volume processed | 2501 ml | Height | 172 cm | |
| Anticoagulant (AC) used | 284 ml | Weight | 62 kg | |
| AC in bag | 19 ml | Total blood volume calculated by cell separator | 4462 ml | |
| SDP volume | 116 ml | Hematological parameters of plateletpheresis donor before and after procedure | ||
| Time taken | 76 min | Pre- | Post- | |
| SDP yield at 15 min | 0.1 × 1011 | Hematocrit | 44.2 | 43.4 |
| SDP yield at 30 min | 0.1 × 1011 | Hemoglobin | 163 gm/l | 162 gm/l |
| SDP yield at 45 min | 0.1 × 1011 | Total Leucocyte Count | 5.3 × 109/l | 5.21 × 109/l |
| SDP yield at 60 min | 0.2 × 1011 | Total Platelet Count | 206 × 109/l | 128 × 109/l |
The trapping of platelets inside the LRS Chamber is a rare event which had never been reported before. We hereby report a new troubleshooting in the Cobe Spectra dual stage ELP collection procedure in the LRS turbo mode. The Cobe Spectra CCM register of the platelet yield during the collection procedure is based on the scattering of red and green light through the plastic cuvette, and the platelet concentration is calculated by a microcomputer, which converts the intensity of the light signal data into a single platelet concentration value.7 The separation of white blood cells from platelets is performed in the LRS chamber and is based on elutriation and leukoreduced platelet movement through the inner tube of the LRS chamber into the collection bag.8 Study quotes increased platelet activation in plasma reduced apheresis platelet concentrate obtained in the LRS chamber in the Cobe Spectra.9 Platelet activation leads to increased p-selectin expression. Adhesion molecules, such as p-selectin, mediate the adhesion of platelets to white blood cells and thus, greater adhesion of platelets to white blood cells (WBCs) inside the LRS chamber may form an obstruction in the collection line.10 Low platelet collection during the apheresis procedure can be explained by the above mechanism and, ultimately, the trapping of platelets inside the LRS Chamber.
Increasing the collection pump flow rate may release the obstruction. Otherwise, the centrifuge must be stopped manually by using the stop spin button on the Cobe Spectra. After opening the centrifuge chamber, the LRS chamber can be tapped gently to release any obstruction. This maneuver should be performed as precociously as possible, once platelet trapping is suspected, based on visual assessment of swirling in the collection bag and the reading of the CCM. A manufacturing defect in the ELP kit may be another reason for the prevention of platelet collection in the collection bag. However, as in our case, the CCM increased slightly after stopping the centrifuge and gently tapping the LRS chamber, ruling out the possibility of a kit defect. Nevertheless, a greater number of observations is required to validate the present troubleshooting and to discover the exact way to overcome the problem, as this event is encountered very rarely or may be under-reported. Moreover, the manual of the cell separator provides no manner with this type of troubleshooting to discover what the problem is, so this should be taken into consideration.
ConclusionClose observation and early detection of the problem may help in the troubleshooting for low-yield platelet collection in the plasma-reduced apheresis platelet collection procedure with leukoreduction in the LRS chamber.
Conflicts of interestThe authors declare no conflicts of interest.