Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoThe management of major bleeding is a critical aspect of modern healthcare and it is imperative to emphasize the importance of applying Patient Blood Management (PBM) principles. Although transfusion support remains a vital component of bleeding control, treating severe bleeding goes beyond simply replacing lost blood. A more comprehensive, multidisciplinary approach is essential to optimize patient outcomes and minimize the risks associated with excessive transfusions.
Severe hemorrhage is the leading cause of death in a variety of clinical scenarios, such as trauma, in the military or with civilians, and high-risk surgeries.1-3 The management of severe hemorrhages involves early recognition of blood loss, control of acute bleeding and replacement of intravascular volume and deficient blood components, generally involving blood transfusion in a regime known as massive transfusion. Delay in starting appropriate transfusion is associated with increased mortality and morbidity.4,5
In trauma patients with acute blood loss, immediate resuscitation, involving the use of blood components, has proven to be important in reducing mortality, bearing in mind that uncontrolled hemorrhage is the cause of up to 50 % of deaths within 24 h after traumatic injury.6
To enable the rapid availability of blood components, trauma centers, in recent decades, have adopted Massive Hemorrhage Protocols (MHP) or standardized criteria for releasing transfusions to arriving patients.7 In fact, the establishment of an institutional MHP has been recommended by several societies.8-10
The objective of this review is to assist healthcare professionals in establishing a MHP for the in-hospital management of adults with critical bleeding resulting from severe hemorrhage.
DefinitionsTo address the topic, some definitions are important:
- •
Critical bleeding
Critical bleeding is a term used to describe a variety of clinical scenarios in which bleeding may result in significant patient morbidity or mortality.
Generally speaking, critical bleeding falls into one of the following categories (which may overlap):
- 1.
Severe bleeding that threatens life and may result in the need for a massive transfusion (transfusion greater than or equal to 5 units of packed red blood cells within 4 h).8,11,12
- 2.
Smaller-volume hemorrhage involving a critical area or organ (e.g., intracranial, intraspinal or intraocular) which may result in patient morbidity or mortality.
- 1.
For this document, the term critical bleeding refers only to the first category.
- •
Massive transfusion
Massive transfusion (MT) is defined based on the volume of blood loss or the volume transfused. There are several definitions proposed by different groups.
For this document, MT is defined as a transfusion greater than or equal to 5 units of packed red blood cells within 4 h.8
- •
Massive Hemorrhage Protocol (MHP)
Given the current context of PBM, preference has been given to using the term critical bleeding management or Massive Bleeding Protocol (MHP), instead of the MT Protocol as it is a more comprehensive, multidisciplinary approach, which involves actions in addition to transfusion support for hemorrhagic control, correction of coagulopathies and normalization of physiological parameters.
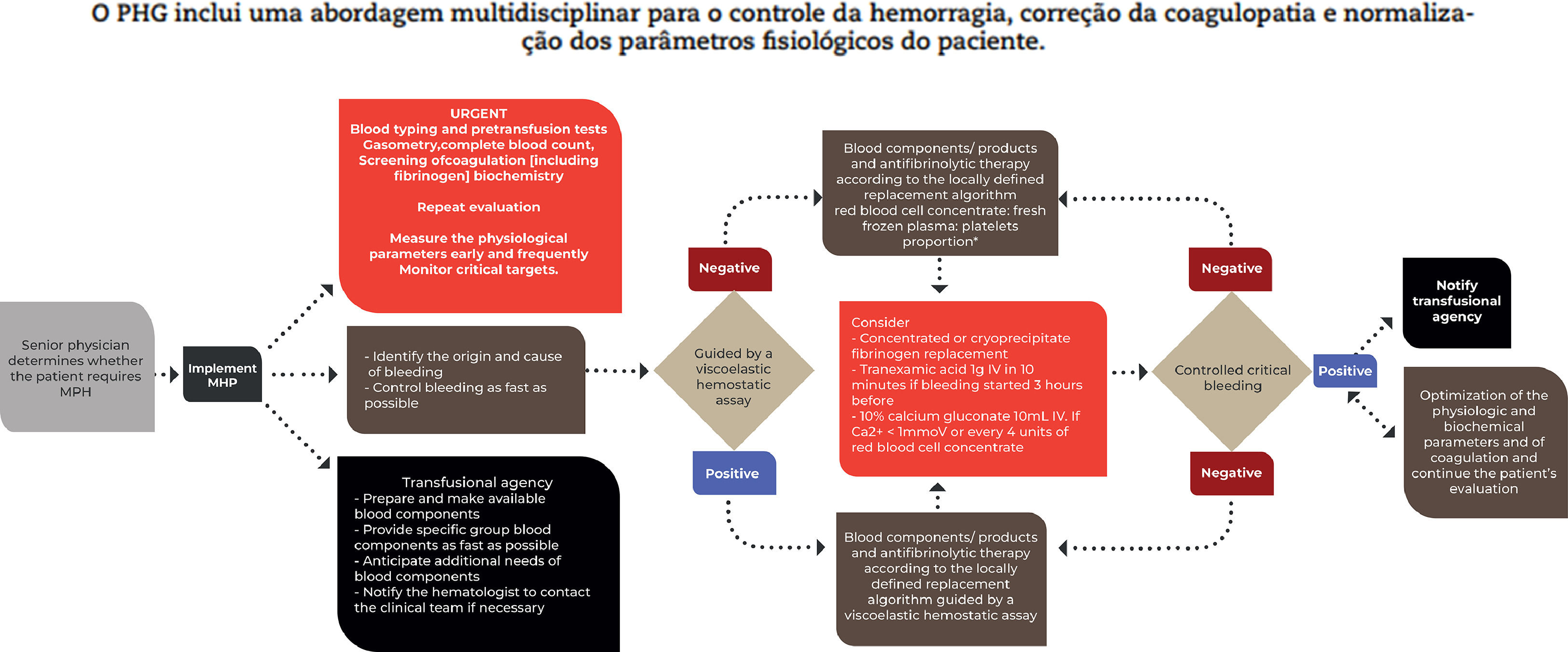
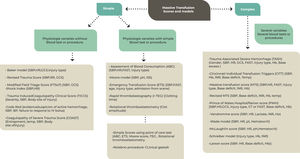
Massive hemorrhage protocol (MHP)A MHP model is suggested in Figure 1.
Adapted from the National Blood Authority, 20238
Some aspects of the MHP deserve special attention and are highlighted in Figure 2.
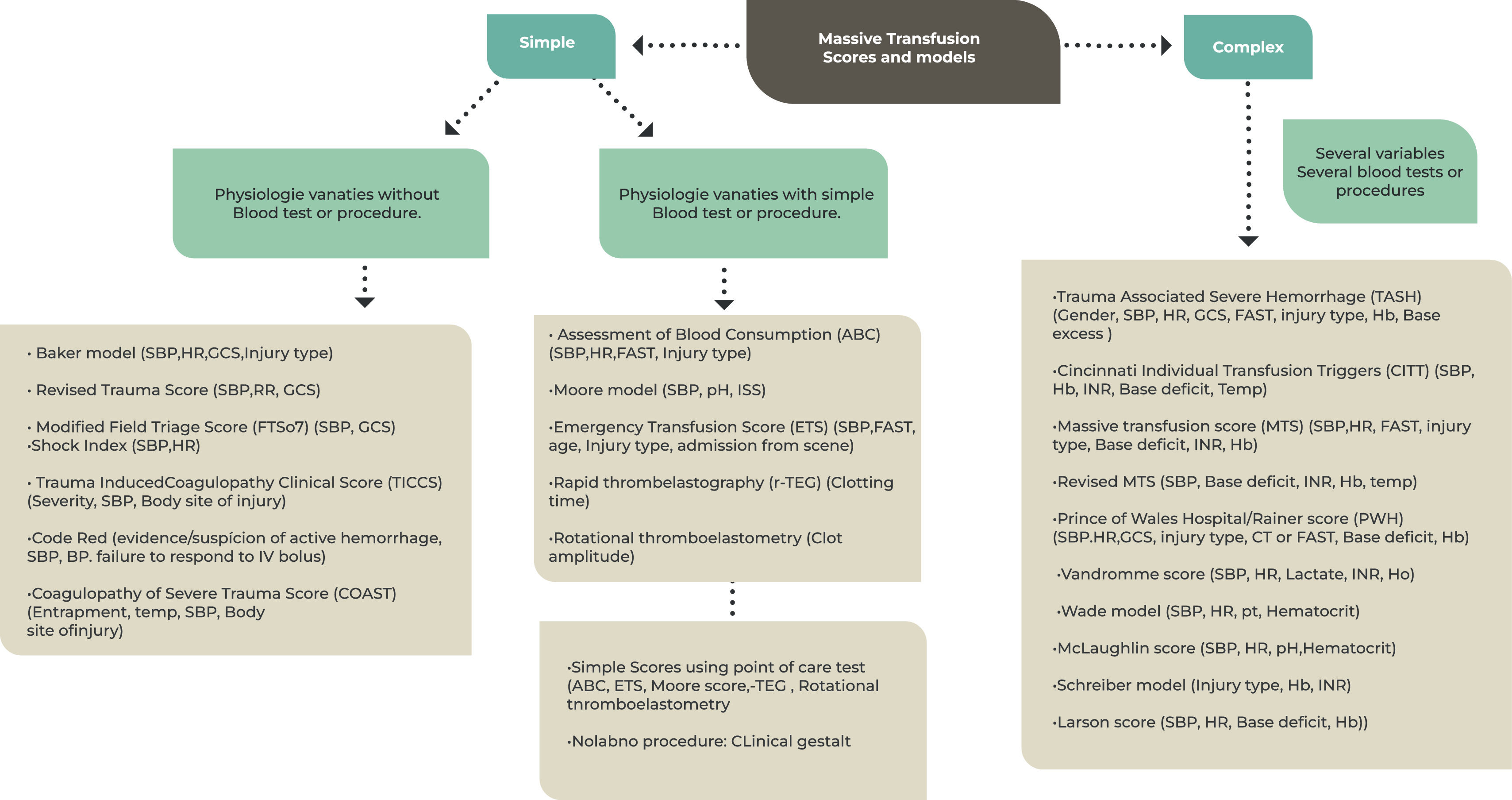
Classification and categorization of the massive transfusion score.
SBP: Systolic blood pressure; RR: Respiratory rate HR: heart rate; Hb: hemoglobin; FAST: focused assessment for the sonography of trauma; BD = Base deficit; GCS = Glasgow Coma Scale; INR: international normalized ratio; ISS: Injury Severity Score. Adapted from El-Menyar et al. (2019)16
MTs are responsible for more than 70 % of the blood transfused in trauma centers.13,14 Even in trauma centers with established MHP protocols, the incidence of over-transfusion can reach 27 %.15 Terminating these transfusions at the appropriate time minimizes the waste of this expensive and limited resource.
Considering the principles of PBM, it is essential to establish guidelines for both the implementation and discontinuation of the MHP aiming at adequate use of blood components and minimizing losses/waste.
The decision to implement a MHP is not an easy one. The decision to implement is often made too early and can result in a waste of blood components.
In this sense, multiple prediction scores and algorithms have been proposed to identify patients in need of MT. Many are complex, requiring difficult calculations and time-consuming laboratory tests, while some are simple and easy to remember, using physiological parameters, lesion characteristics and/or simple procedures such as point of care. The variables most commonly used in these models include: systolic blood pressure (SBP), heart rate (HR) and hemoglobin level (Hb). The results of base deficit, serum lactate, international normalized ratio (INR) and focused assessment for the sonography of trauma (FAST) are also used as covariates in some models.16
There is no consensus on a specific perfect score. They all lack prospective validation and each scoring system has its advantages, disadvantages and most useful scenarios, which affect their applicability on a broad scale.9,16
Some factors need to be considered when deciding to implement a MHP, such as the cause of hemorrhage and the rate of blood loss, the mechanism of trauma (if any), the current physiological state of the individual and the likely need for continued support using blood components. Thus, it is essential to designate the professional(s) who will be responsible for implementing the MHP at each center. Most groups determine that this role falls to the senior physician on the team.
The decision to interrupt a MT should be made jointly among the doctors on the resuscitation team and should be communicated immediately to the blood bank. Some groups suggest that the interruption of MT should take into account anatomical criteria (bleeding stopped through surgical control or angioembolization) and physiological criteria (stable or increasing blood pressure and improvement or stability of target organ perfusion measurements).13,17,18 When achieved, MHP should be discontinued and, if the patient still requires resuscitation, transfusion therapy should be goal-directed.
Interrupting MT is also justifiable and necessary when the care team determines that there is no longer any benefit for the patient in question, when the injury or process is considered irreversible. However, early recognition of the futility of MT is a challenge and current studies have not yet identified variables that can accurately determine the risk of early mortality.19
Initial clinical assessment, and physiological, biochemical and metabolic parametersIn the event of critical bleeding, it is essential to identify the cause of the bleeding and control it as quickly as possible. Common causes of critical bleeding include trauma, gastrointestinal bleeding, ruptured aortic aneurysm, obstetric hemorrhage and surgical procedures. The first signs of blood loss are not always recognized however, significant blood loss triggers a sequence of physiological responses to maintain cardiac output and preserve blood flow to vital organs. Thus, physiological changes and biochemical parameters can be used to recognize a critical hemorrhage.
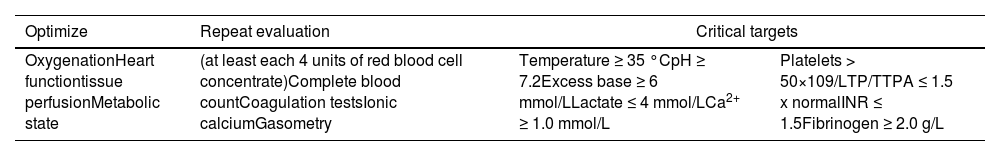
In patients with critical bleeding who require MHP, it is recommended that, in addition to monitoring physiological parameters, the following parameters are measured early and frequently:*8
- •
Temperature
- •
Acid-base state
- •
Ionic calcium
- •
Hemoglobin/platelet count
- •
Prothrombin time (PT)/INR/Activated partial thromboplastin time (APTT)
- •
Fibrinogen
- •
Temperature ≥ 35 °C
- •
pH ≥ 7.2
- •
Base deficit ≥ −6 mmol/L
- •
Lactate ≤ 4 mmol/L
- •
Ca2+ ≥1.0 mmol/L
- •
Platelet count > 50 × 109/L
- •
PT/APTT ≤ 1.5
- •
Fibrinogen ≥ 2.0 g/L
*Some groups suggest repeating these parameters every four units of packed red blood cells transfused. This periodicity must be defined and adapted locally.
Proportion between red blood cells and other blood components, time and doseIn the MHP, red blood cells and other blood components are made available in ‘transfusion packages’ following fixed or pre-defined ratios of red blood cell concentrate (RBCC): fresh frozen plasma (FFP): platelets (PLT). The most commonly used ratios are 1:1:1 and 2:1:1 of RBCC:FFP:PLT, respectively.
Studies that evaluated the impact on mortality of using ratios 1:1:1 (high) and 2:1:1 (low) in the transfusion of patients with critical bleeding showed conflicting results15,17,20-24 and therefore there is no way to define the best RBCC:FFP:PLT ratio.
Most groups suggest using at least the 2:1:1 ratio of RBCC:FFP:PLT. The choice and adaptation of this proportion must take into account local characteristics such as stock, storage availability, preparation time, durability of blood components (mainly platelets), among others.
An example of a 1:1:1 package would be four units of RBCC, four units of FFP, and four units of whole blood donor-derived platelets*. In the package with a 2:1:1 ratio the RBCC would be doubled (eight units).
*The transfusion unit available in each center may be different. For example, centers may make platelets derived from apheresis collection available, where one platelet apheresis unit generally corresponds to six units of platelets derived from whole blood donors. Therefore, just like the proportion used, the constitution of the packages must also be adapted to the local reality.
Blood components and productsBlood components refer to RBCC, FFP, PLT as discussed in the previous item, and cryoprecipitate (CRIO).
Blood products refer to plasma derivatives or plasma-derived proteins, such as fibrinogen concentrate and prothrombin complex concentrate, resulting from the fractionation of large amounts of human plasma. Fibrinogen is a key coagulation protein required for the formation of stable clots and is the first coagulation factor to reach critically low levels during bleeding. Replacement can be done using CRIO or fibrinogen concentrate where the most appropriate alternative must be defined locally depending on availability and cost etc. There is not enough evidence to establish the best time or optimal dose for fibrinogen replacement during MHP. In most protocols, this replacement is guided by the results of laboratory coagulation tests or viscoelastic hemostatic assays (VHAs).8
It must be considered that, in some centers where thawed plasma is not kept, the FFP transfusion may not be started at the same time as the RBCC transfusion, resulting in significant delays in obtaining the RBCC:FFP ratio.25 During this interval, the fibrinogen level is likely to be lower than desired. Therefore, some centers use fibrinogen concentrate to quickly restore fibrinogen levels.9,26
The prothrombin complex concentrate is reserved for reversing the action of coumarin anticoagulants at a dose of 25–50 IU/kg.8AntifibrinolyticsTranexamic acid has been used in trauma patients in the pre-hospital context or started within three hours after the trauma (at a dose of 1 g, with a second administration at the same dose in eight hours) after the publication of the results of the CRASH-2 study. 27 The decrease in the early mortality rate led to the inclusion of tranexamic acid on the World Health Organization Model List of Essential Medicines for the treatment of trauma in 2011. 28 A recent study (PATCH-Trauma) showed similar results in reducing the mortality rate in 24 h and 28 days, without evidence of an increase in thrombotic events in a group using tranexamic acid. 29 Therefore, most groups recommend the administration of tranexamic acid (up to three hours after injury) as part of the MHP in patients with critical bleeding.
The use of tranexamic acid in women with postpartum hemorrhage is recommended by some groups due to the results of the WOMAN study, which showed a lower rate of mortality related to bleeding in the group of patients who received the medication (mainly less than three hours after the onset of bleeding). The tranexamic acid regimen used was 1 g, which could be repeated in 30 min (if the bleeding was uncontrolled) or in 24 h (if rebleeding).8,30
A possible benefit of using tranexamic acid has been suggested for gastrointestinal (GI) tract bleeding, particularly upper GI tract bleeding. 32 Recently, however, the HALT-IT randomized study, involving 12,009 patients with GI tract bleeding, did not show a reduction in the mortality rate in patients who received tranexamic acid when compared to those who received placebo. 31 Therefore, there is no evidence to support the use of antifibrinolytics in patients with GI bleeding.
Viscoelastic hemostatic assay (VHA)VHAs are tests that provide, from a whole blood sample, a functional assessment of clot formation, clot strength and its degradation. VHAs can be used in critically bleeding patients to assess coagulopathies and guide antifibrinolytic and blood component/product therapy as part of a MHP. Interpreting the results requires specific knowledge and training.
In the setting of critical bleeding management in the MHP, evidence is limited in showing superiority of results for transfusion guided by VHAs over transfusion guided by conventional coagulation tests. Furthermore, the use of these tests involves availability, logistics, training and costs that must be adapted to the reality of the institution. Some societies, such as the Australia Society, suggest that VHA, if used in this context, should be used in conjunction with an established MHP.8
Massive hemorrhage protocol (MHP) adaptationThe MHP must be adapted to local institutional needs and resources (access to blood components/products/medicines, stock, transportation, distance between the care service and the service responsible for transfusion support, time for preparation, laboratory support and ease of communication with the blood therapist /hematologist). The very definition of the proportion of blood components for transfusion and the constitution of transfusion packages need to be adapted to local conditions, as discussed previously. Furthermore, the MHP can be modified to address specific populations such as obstetric patients, with the potential for occult hemorrhage and the early development of disseminated intravascular coagulation (DIC), for example.
Adverse eventsTransfusions are not without risk. The risk of transfusion reactions must be considered when utilizing a MHP. Complications such as transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), transmission of infectious diseases (including prion diseases), ABO incompatibility, and allergic reactions can contribute to poor patient outcomes.
Therefore, the decision to transfuse must take into account the entire range of available treatments, evaluate the evidence of effectiveness against the risks associated with transfusion and, finally, consider the patient's values and choices.
Recommendations
- •
A multidisciplinary approach must be used for critical bleeding, aiming at bleeding control, correction of coagulopathies and normalization of physiological parameters.
- •
It is essential to identify the cause of the bleeding and control it as soon as possible.
- •
Critical bleeding should not be managed with transfusion alone.
- •
It is recommended that a MHP be established to care for patients with critical bleeding.
- •
The MHP must be adapted to local institutional needs and resources.
- •
It is essential to establish effective communication between the transfusion agency and the care team.
- •
Implementation of the protocol must be carried out by an experienced professional defined in advance, as there is no consensus on the best predictive score for severe bleeding and the need for massive transfusion.
- •
Interrupting the MHP protocol at the correct moment and immediate communication to the transfusion agency are essential to minimize losses and the best use of blood components.
- •
Implementation of the MHP must be carefully weighed as transfusions are not without risk.
In conclusion, the adoption of a Patient Blood Management (PBM) approach to the treatment of serious bleeding highlights a fundamental shift in healthcare towards more efficient and patient-centered care. By prioritizing meticulous bleeding control, comprehensively addressing coagulation challenges and promoting multidisciplinary collaboration, PBM not only increases patient safety but also minimizes excessive dependence on blood products and transfusions. This conscious use of blood products and exploration of viable transfusion alternatives not only reduces the risks associated with transfusions, but also optimizes resource allocation. In the evolving landscape of healthcare, PBM stands as a beacon of comprehensive and insightful care, ultimately providing superior outcomes for patients facing severe bleeding scenarios.