Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoAutologous blood transfusion can be achieved through different techniques, including by the patient donating blood before surgery (pre-deposit), collecting blood from the patient immediately before the operation and replacing the volume with colloids or plasma expanders (acute normovolemic hemodilution) or through the salvage of lost blood, during or immediately after surgery, and its retransfusion after washing (intraoperative or postoperative recovery).
We will focus on the two methods used intraoperatively that are of fundamental importance in the management and conservation of the patient's own blood.
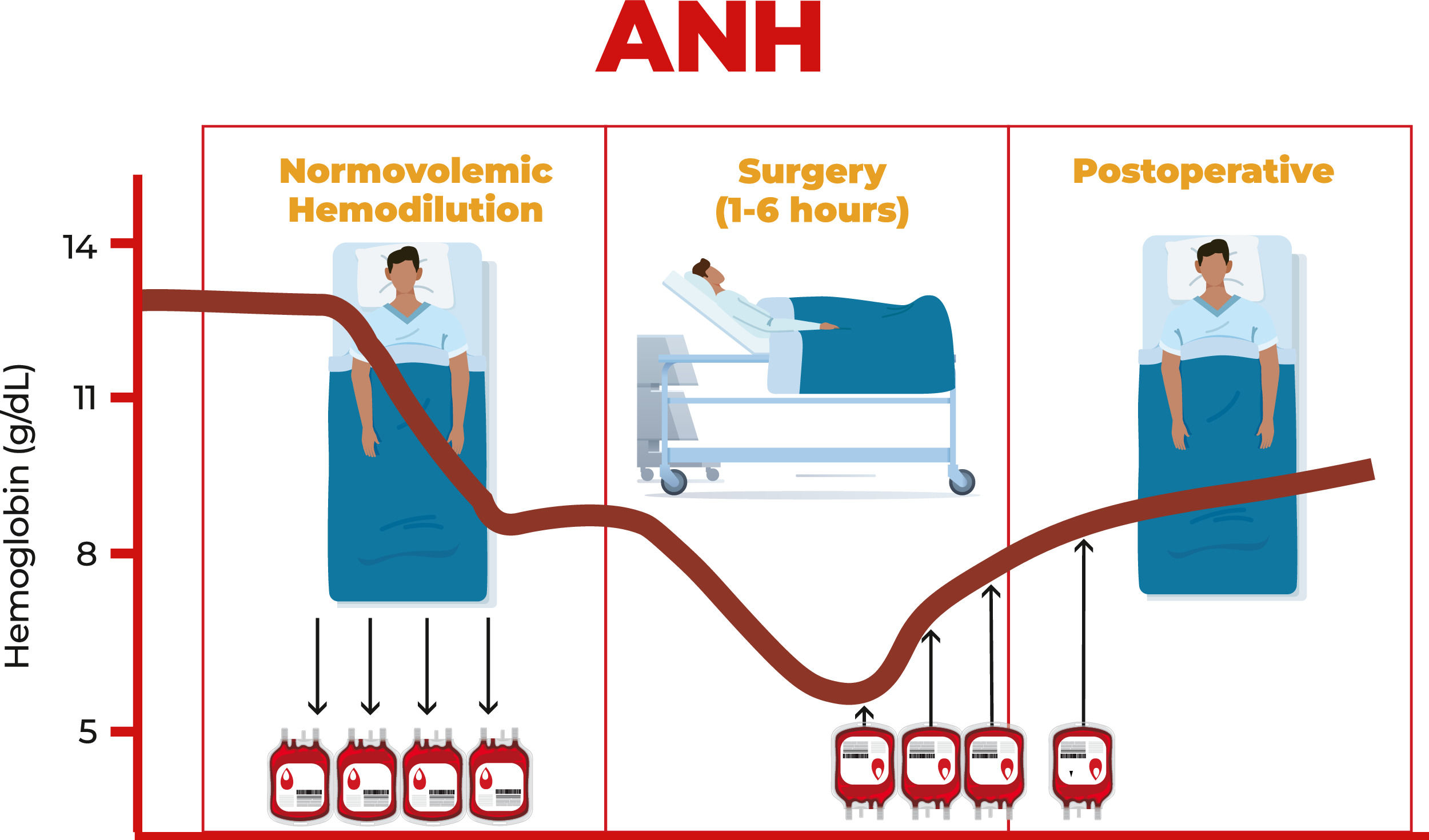
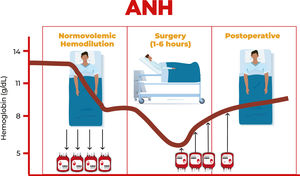
Acute normovolemic hemodilution (ANH) was introduced in the 1970s and initially used in cardiac surgeries.1 However, aiming to conserve the patient's own blood, its use has been expanding to several other surgical areas that encompass great blood loss. ANH is a simple and low-cost procedure, with no evidence of clotting, hemolysis, fibrinolysis or immunological activity in the collected blood.2 It is the only technique capable of collecting ‘fresh whole blood’.
ANH is a technique that involves, with due monitoring, the removal of blood from the patient in the operating room immediately after anesthetic induction and replacing it with crystalloids and/or colloids to maintain normovolemia Figure 1.
The amount of blood withdrawn varies between one to three units (450 to 500 mL each) and this blood is retransfused during or within a period of eight hours after surgery.
There is a formula proposed by Gross to assist in the calculation of the quantity that can be withdrawn:
Where:
V = Volume of blood to be withdrawn
EBV = Estimated blood volume of patient (generally 70 mL × patient's weight in Kg)
Hi = Initial hematocrit
Hf = Final hematocrit
Hav = Average hematocrit of the procedure
Each blood unit must be labeled with the patient's name, registration number, time of blood withdrawal and sequence number (if more than one unit is withdrawn). It is important to remember that the reinfusion of whole blood units returns red blood cells, clotting factors and platelets back to the patient and must be done in reverse order, that is, the last bag collected is the first to be transfused, due to the factors of coagulation and platelets that will be in higher concentrations/more active.
The objective of ANH is to reduce the concentration of red blood cells in the patient's circulation, thus reducing blood loss during the surgical procedure and at the same time maintaining normal circulatory volume and preventing anemia. It also reduces the need for transfusions of allogeneic red blood cell concentrates.
There are many other possible beneficial effects of ANH, including reduced risk of adverse events such as reactions and infections related to allogeneic blood transfusions, less red blood cell damage, less impact on coagulation, preservation of red blood cell and platelet mass, as well as improved perfusion during extracorporeal circulation (CPB), resulting in increased oxygen delivery to tissue.3 In patients with normal or elevated initial hemoglobin levels undergoing cardiac surgery, the blood viscosity associated with induced anemia may have cardioprotective effects.4
It is important to note that ANH is not suitable for all patients. Patients with pre-existing anemia and those who cannot tolerate reduced blood volume may not be ideal candidates. Therefore, low ejection fraction (<45%), renal failure with oliguria and low baseline hemoglobin (<11 g/dL) are contraindications for ANH.
A relevant limitation of ANH is the lack of knowledge of the technique by professionals, especially by anesthetists who are not accustomed to its use and, thus, many resist using this technique. The decision to perform ANH should be based on a thorough assessment of the patient's medical history, condition, and potential risks and benefits.
A 2017 meta-analysis of randomized studies limited to cardiac surgery, which compared ANH and cases where the technique was not used, found reductions in allogeneic transfusions.3 Another meta-analysis from 2015 found a 25% reduction of allogeneic transfusions in cases in which ANH was used compared to its non-use in any type of surgery.5 A different publication from 2020 on major orthopedic surgeries, demonstrated that the need for allogeneic blood was significantly lower in the ANH group compared to a control group and postoperative complications were significantly lower in the ANH group.6
Positive results were also found in a retrospective study from Japan. Saito et al. evaluated the effectiveness of ANH in reducing perioperative transfusions in 586 patients with gynecological cancer. The results of this study demonstrated a significantly lower perioperative transfusion rate in the group that underwent ANH compared to the group that did not (3.5% vs. 11.8%; p-value <0.001).7 Another study from the University of Sao Paulo in Ribeirão Preto (HC-FMRP-USP) carried out a retrospective survey of surgery for the elective correction of scoliosis with and without the use of ANH. The results were a reduction in the volume of allogeneic blood used by the hemodilution group. The percentage of patients who required transfusion was 12.5% in the hemodilution group, while in the control group it was 70.69%.8
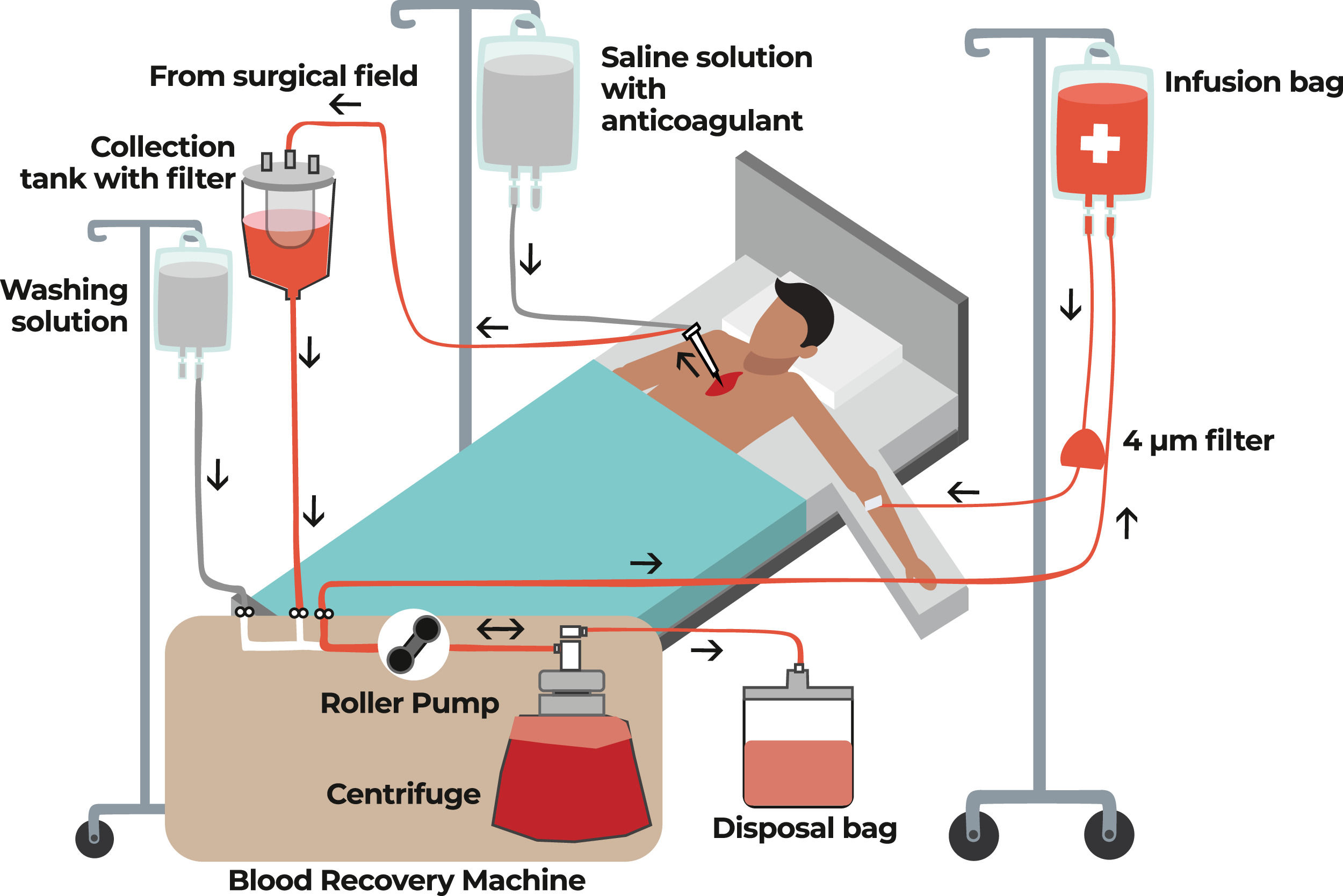
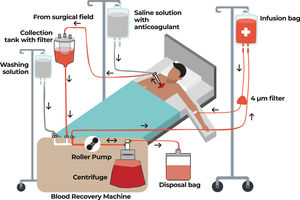
Intraoperative autotransfusionThe recovery of intraoperative autologous blood (minimizing blood losses) is a very important tool and is one of the main focuses in the second pillar of Patient Blood Management (PBM). This is achieved with a cell saver, also known as cell salvage equipment, which is mainly used in major surgeries, such as heart surgeries, but its use is expanding to other types of surgery Figure 2.
The process of collecting, filtering and cleaning blood from the surgical field in order to have autologous blood for transfusion back to the patient is known as intraoperative blood recovery. Red blood cells are retained, but plasma, platelets, heparin, free hemoglobin, and inflammatory mediators are discarded along with the saline solution. The equipment used in this process extracts blood, which is then combined with heparinized saline solution or citrate anticoagulant. Blood aspirated from the surgical field is returned to the patient during surgery as autologous blood.
In the recent past, some heart surgeons were not adept at using intraoperative recovery due to the belief that the heparin used in the equipment during the process could cause some type of bleeding. However, it has been demonstrated that residual heparin concentrations in the final product are clinically insignificant and do not increase the risk of intra- or post-operative hemorrhage.9
Patients with estimated intraoperative blood loss of at least 500-1000 mL or between 10%10 and 20% of blood volume are indicated for the use of intraoperative recovery.
The use of intraoperative recovery is traditionally contraindicated in cases of generalized infection, surgical site infection, neoplasms and in surgeries to resect tumors, but there is increasing evidence to support its use even in these scenarios. With the use of a leukodepletion filter, there is a 99% reduction in bacterial contamination.11 Furthermore, the minimal risk of bacterial infection must be weighed against the increased risk of infection from secondary immunomodulation that can be triggered by an allogeneic blood transfusion.12
Although recovered autologous blood is an option for preoperative blood conservation in oncological surgeries, it is often not used due to concerns about reinfusing tumor cells and, thus, causing tumor dissemination. Studies have not shown an association between the use of intraoperative recovery and the spread of metastasis during cancer surgery.13,14 Therefore, these findings open a new perspective in the indication of intraoperative autologous recovery.
Another group of patients for which the use of intraoperative autotransfusion has increased are pediatric patients undergoing cardiac surgery. It was a population in which salvage techniques were traditionally excluded based on the thought that the volume recovered would not justify the expense. This stance has changed with evidence of decreased necessity of transfusion and is part of a blood conservation strategy, as autologous concentrates can be transfused as needed without the risks associated with allogeneic transfusions. It is also worth mentioning that different sizes of bowls exist that are suitable depending on the patient's weight to achieve maximum recovery without affecting performance related to the patient's size. There is growing published evidence supporting the use of blood salvage in pediatric heart surgery as a component of blood conservation programs.15-17
In a meta-analysis, Wang et al.18 showed that the use of intraoperative blood salvage reduced the exposure rate to packed red blood cells, without having any effect on the number of transfusions of fresh frozen plasma or platelet concentrate. Likewise, a Cochrane review in 2010 demonstrated that the risk of transfusion was reduced by an average of 34% in patients undergoing heart surgery with the use of intraoperative blood recovery.19 The 2014 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization advocate the use of cell salvage. 20 Its use is also recommended by the American Society of Thoracic Surgeons (STS) and the Society of Cardiovascular Anesthesiology (SCA) as a routine procedure (Class I, Level of Evidence A).21
Most cell saver machines currently available on the market have ‘semi-continuous’ blood flow, in which each phase of the process works in isolation. This characteristic allows for enormous interaction with perfusion/extracorporeal circulation, but must be viewed with caution, as the machine operator can interfere in these phases and, consequently, obtain a low-quality concentrate of washed autologous blood cells. It is therefore recommended that operators participate in monitored training and qualification programs and, above all, that quality control of this final product, which will be reinfused into the patient, is carried out.
Recommendations
- •
We recommend the use of acute normovolemic hemodilution and cell salvage in cases of large and complex surgeries, especially in surgeries with a high risk of significant blood loss.
- •
It is essential that the medical and nursing teams are properly trained and familiar with the appropriate use of techniques to ensure the safety and effectiveness of the procedure.
For intraoperative blood recovery strategies to be effective, with the aim of eliminating inappropriate and unnecessary allogeneic transfusions, it is necessary for hospitals and health institutions to develop a program, an infrastructure, and a focused and trained team that promotes changes in the traditional clinical practice. Today this is achieved through an understanding of Patient Blood Management (PBM) which is currently defined as a patient-centered, systematic and evidence-based approach to improving patient outcomes by managing and preserving the patient's own blood and at the same time promoting safety and empowerment.