Acute myeloid leukemia (AML) with t(8;16)(p11;p13)/KAT6A::CREBBP is a very rare condition that accounts for less than 0.1 % of de novo AML cases.1 According to the European LeukemiaNet (ELN) 2022 recommendations presence of t(8;16) abnormalities is a cytogenetic predictor of dismal prognosis and short survival.2 Due its rarity, not more than 100 cases have been reported so far. Clinical course is found to be aggressive with frequent extramedullary involvements (e.g., leukemia cutis, myeloid sarcoma), the presence of disseminated intravascular coagulation (DIC) and a high likelihood of early relapse. Myeloid blasts often present monocytic or myelomonocytic differentiation with the lack of CD34 and CD117 expression making laboratory diagnosis challenging.3,4 Herein, we present a fatal case of a young male with AML and t(8;16).
Case reportAn 18-year-old male patient was diagnosed with AML in July 2022. On admission he complained about headaches, night sweats and subfebrile body temperature for a few weeks. Physical examination revealed isolated bruises and pale-pink merging skin lesions on shoulders and back. Additionally, supraclavicular lymphadenopathy of approximately 2 cm was present. His-blood count presented an elevated leukocyte count (WBC 68 × 109/L) with moderate anemia (Hb 9.5 g/dl) and thrombocytopenia (PLT 47 × 109/L). Blood smear showed 52 % of blasts. Coagulation tests were in line with the features of DIC.
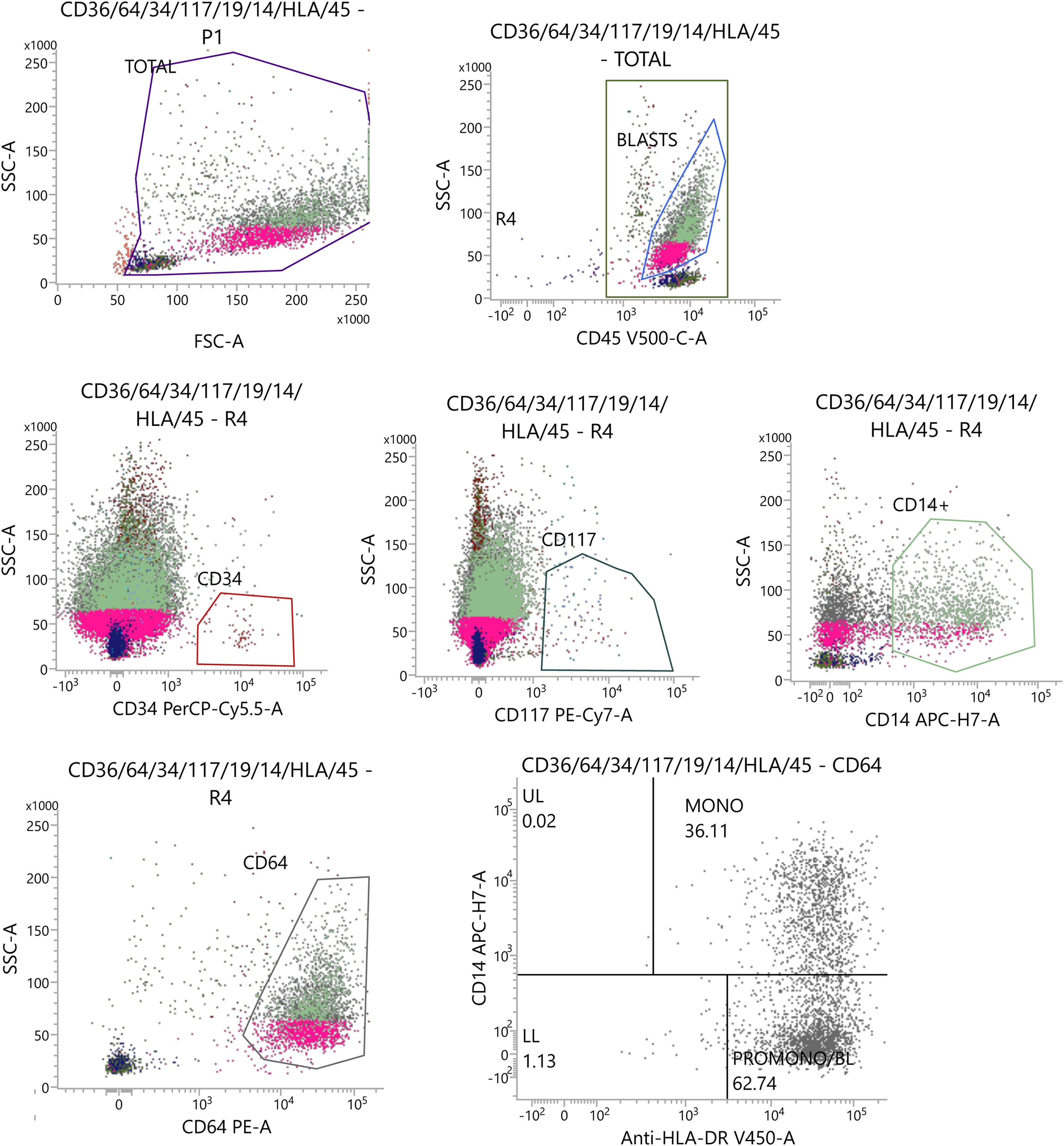
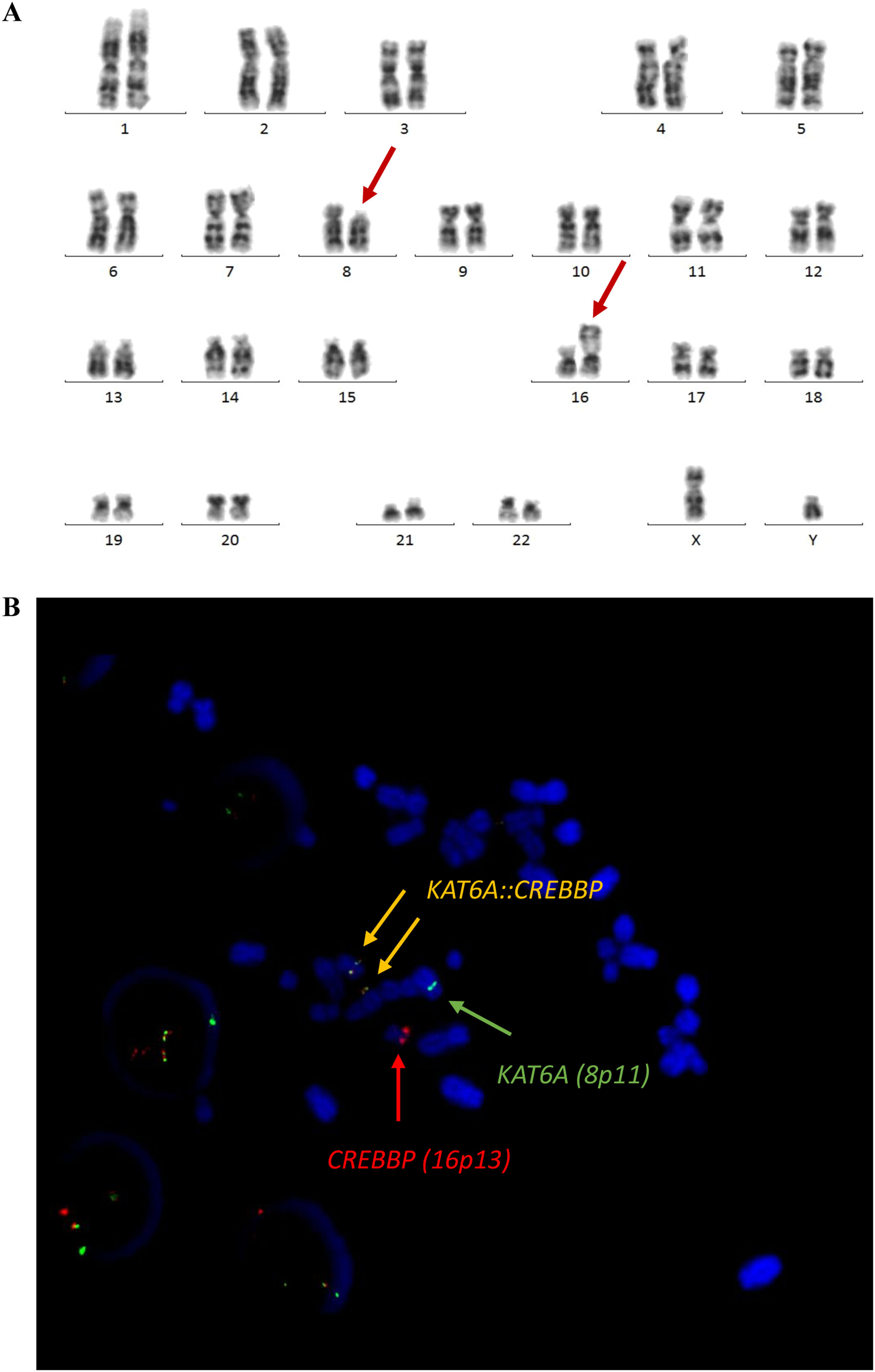
Cytologic examination of bone marrow aspirates showed almost complete infiltration with leukemic cells. Multi-parameter flow cytometry of bone marrow samples detected 75 % of monoblasts with the immunophenotype as follows: CD45++, CD34-, HLA-DR+, Tdt-, CD38+, CD13+, CD33+, CD117-, CD15+, CD65+, MPO, CD11b+, CD14+, CD64+-, CD36+, CD7-, CD2-, CD56+, CD4+ (see Figure 1). The NPM1, FLT3-ITD, FLT3-TKD, CBF::MYH11, RUNX1::RUNXT1, MLL::MLLT3 and BCR::ABL(p210) were not present on molecular assay using reverse transcriptase-polymerase chain reaction (RT-PCR). For cytogenetic examination 24-hour and 48-hour G-CSF-stimulated cultures were performed. Cytogenetic preparations were stained using the GTG technique. Evaluated karyotype revealed presence of t(8;16)(p11;p13) translocation. Fluorescence in situ hybridization (FISH) with a two-color, dual-fusion probe for green-labeled KAT6A (8p11) and red-labeled CREBBP (16p13) (CytoTest, Rocville, MD) confirmed the presence of KAT6A::CREBBP fusion transcript as presented on Figure 2.
Cytogenetic findings. (A) Karyotype analysis: 46,XY,t(8;16)(p11.2;p13.3) (B) FISH Probe Kit Translocation analysis using Dual Fusion KAT6A-CREBBP CytoTest: KAT6A (green)/CREBBP (red) showing 2 fusion signals (orange), indicating ish t(8;16)(KAT6A+,CREBBP+;CREBBP+,KAT6A+) rearrangement.
Patient initiated an induction regimen with daunorubicin and cytarabine (3 + 7). Post-treatment aplasia was complicated by neutropenic fever and severe abdominal pain with diarrhea of unknown origin. Clostridioides difficile infection was excluded. Bone marrow aspirate performed after induction met hematologic criteria of complete remission. Measurable residual disease (MRD) was not assessed due to lack of aberrant immunophenotype. The patient had two sisters but neither of them was HLA-identical and therefore a search for a matched unrelated donor was initiated. Simultaneously, he started the first course of consolidative treatment with high doses of cytarabine. The treatment went without any remarkable complications.
On admission to the second consolidative therapy, the patient presented the features of thrombocytopenic diathesis on lower limbs and back. Blood count revealed leukocytosis of 33 × 109/L with severe thrombocytopenia (PLT 30 × 109/L) and mild anemia (Hb 10.5 g/dl). On blood smear 36 % of monoblasts were present. Additionally, coagulation tests showed low fibrinogen level (0.52 g/l) with prolonged prothrombin time of 21.3 s and elevated d-dimers concentration (30 289 ng/ml). Leukemia relapse was evident, and he started re-induction treatment with cladribine, cytarabine and mitoxantrone (CLAG-M). A week later, the patient started to complain of a severe headache with concomitant nausea and vomiting. A computed tomography (CT) was consistent with a fresh intracerebral bleeding at the right parietal lobe with the presence of subdural hematoma at the left frontal lobe. Due to his thrombocytopenia and coagulopathy, he was disqualified from neurosurgical intervention. While he continued a supportive care his general condition was deteriorating with intermittent seizures. A progression of intracerebral bleeding with generalized cerebral oedema was demonstrated on subsequent CT scans. Finally, patient died 2 weeks after reinduction chemotherapy.
DiscussionIn many aspects the presence of t(8;16)(p11;p13)/KAT6A::CREBBP is associated with an unique subtype of AML. Firstly, flow cytometric analysis of bone marrow aspirates often reveal distinctive immunophenotypic profile with bright expression of CD45, remarkably high side scatter and lack of CD34 and CD117 expressions.4 Myeloid blasts usually present myelomonocytic phenotype with the presence of CD13, CD33 and CD64. Lack of CD34 and strong expression of CD33 as well as clinical course of the disease with DIC may be misleading and suggest the diagnosis of acute promyelocytic leukemia. However, cytologic picture is more characteristic for AML and often presents monoblasts with cytoplasmic vacuolation and erythrophagocytosis.3 Some authors report that t(8;16) is prevalent for therapy-related AML.3,4,5
Appropriate MRD assessment of t(8;16) AML may also be challenging. Fusion of the KAT6A gene at chromosome 8p11 with CREBBP gene located at chromosome 16p13 is proved to result in a variety of fusion transcripts due to alternative splicing.6 Identification of the right transcript type may be crucial for accurate molecular MRD assessment, especially after allogeneic hematopoietic stem cell transplantation (allo-HSCT).7
Long-term follow-ups on t(8;16) AML are limited to a few publications. A large analysis with 15 patients at median age of 50 years demonstrated an unfavorable outcome with overall mortality of 60 % after median follow-up of 18.2 months. All patients received intensive induction treatment but only five of them proceeded to allo-HSCT. It was suggested that patients diagnosed with de novo t(8;16) AML had a superior survival compared with t(8;16) therapy-related AML (t-AML). Moreover, better outcomes were demonstrated for patients with t(8;16) as a sole abnormality vs t(8;16) with two or more additional karyotypic changes. Three patients presented extramedullary infiltrations and 3 more were reported to have DIC.5 Another report of 5 patients (all of them with t-AML) showed fatal DIC for 3 of them after median of 10 days. Two other patients died after allo-HSCT for leukemia recurrence.4 To sum up the data presented above, it is obvious that only approximately 35 % of adult patients with t(8;16) AML are able to survive until allo-HSCT, which is the only potentially curative therapeutic option. However, prognosis seems to be better in the newborns. Unlikely to adults, the KAT6A::CREBBP fusion gene is a frequent molecular finding in congenital leukemia with 50 % incidence of spontaneous remissions within 2–4 months from diagnosis.8,9 Infants that require chemotherapy typically demonstrate a favorable response to standard treatment.10
ConclusionsOur patient presented many features of t(8;16) AML which were in line with those presented by abovementioned authors. One should keep in mind that diagnosis may be challenging, DIC predominates the initial clinical picture and resistant to chemotherapy is common.