Brazil is one of the countries with the largest population of people with hemophilia (PwH) worldwide. In this scoping review, we aim to investigate the Brazilian context for hemophilia regarding three predefined concepts: (i) clinical-epidemiological profile, (ii) burden of disease and (iii) patient journey and unmet needs.
MethodsThree questions in each concept guided the screening of references retrieved by systematic searches carried out in MEDLINE, LILACS and the Digital Library of Theses and Dissertations. Quantitative and qualitative studies conducted in Brazil from 2002 onwards were assessed for eligibility.
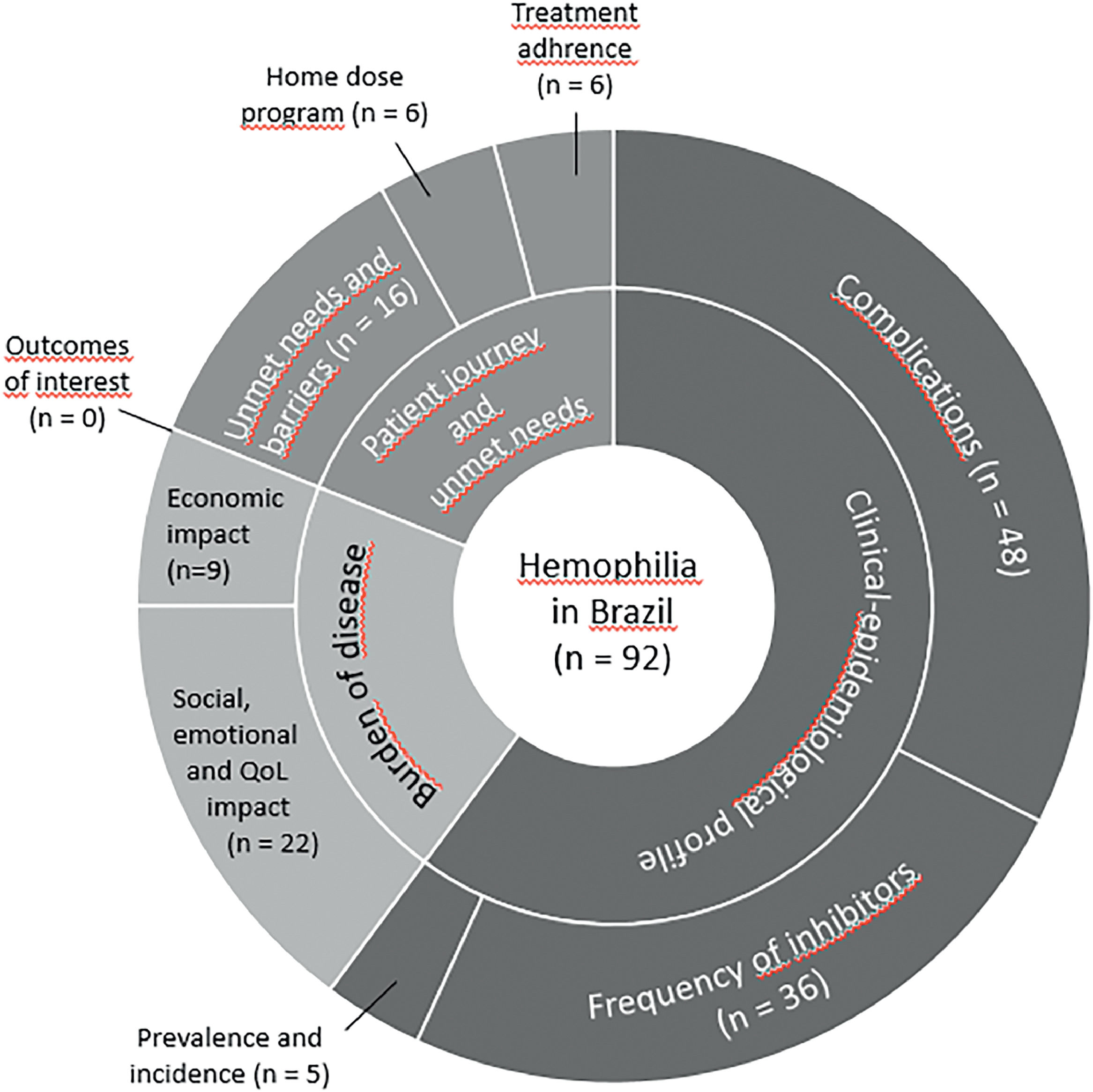
Main resultsNinety-two studies were included. A total of 66 studies addressed the concept “Clinical-epidemiological profile”, 31 investigated the concept of “Burden of disease” and 26 addressed the concept “Patient journey and unmet needs”. Based on these studies, pain and arthropathy affect a substantial proportion of the PwH, with physical functioning, pain and school or work being the domains of quality of life with the greatest impact. About 43 % to 82.6 % of the PwH are unemployed. Rates of inhibitor development are highly variable across studies, especially in hemophilia A. Adherence to prophylactic treatment ranges from 25 % to 72 %. The annualized bleeding rate is estimated at 2.4 ± 4.1. The barriers to treatment identified include distance to reference centers, lack of coordination of specialized and emergency care and restricted access to rehabilitation.
ConclusionsHemophilia poses a considerable burden on the PwH. Despite the available modalities of treatment, there are remaining unmet needs that should be addressed by researchers and policy makers in the future.
Hemophilia A (HA) and hemophilia B (HB) are a rare group of X-linked recessive hemorrhagic diseases, being characterized by a deficiency or abnormality in the clotting factors VIII and IX, respectively. HA and HB are marked by bleeding manifestations, including spontaneous bleeding episodes commonly into muscles and joints. Joint bleeding usually affects weight-bearing joints, such as knees or ankles, which can lead to the development of disabling hemophilic arthropathy.1 In addition, people with hemophilia (PwH) are also at risk for life-threatening bleeding, including intracranial hemorrhage.1
Advancements in hemophilia treatment have markedly shifted the disease from being a life-threatening condition to a chronically manageable one. Central to this progress is the institution of prophylactic treatment, which entails routine administration of clotting factor concentrates (CFCs) to avert bleeding episodes. Specifically, factor VIII is utilized for HA, and factor IX for HB. Prophylactic therapy mitigates the risks of joint damage and other complications stemming from recurrent bleeding, thereby enabling the PwH to have a more active lifestyle. Prophylactic therapy also leads to fewer hospitalization episodes and a decrease in school or work absenteeism, thus considerably reducing the disease's burden on both the PwH and their families.2
In Brazil, the public health system provides access to therapies in addition to CFCs, including immune tolerance induction, bypass agents (recombinant factor VIIa) and emicizumab, which are particularly relevant for treating the PwH who develop inhibitors.3,4 Extended half-life products have the potential to enhance the landscape of prophylactic treatment in hemophilia, due to less frequent dosing and tailored treatment regimens, although this modality of treatment is partially available in the public health system or Unified Health System in Brazil. Despite the available therapies, hemophilia places a significant burden on patients/caregivers, payers and society. It results in direct costs from hospitalizations, outpatient visits and drug treatments, as well as in indirect costs from diminished work productivity and absenteeism. Hemophilia also presents intangible costs, including impaired quality of life, pain and suffering of the inflicted and their families and the emotional and physical impact on them and their caregivers.2
According to the 2020 Annual Global Survey of the World Federation of Hemophilia,5 Brazil is one of the countries with the largest population of PwH. Despite the high prevalence and burden of hemophilia in Brazil, little information is available regarding certain aspects, such as the burden of disease and determinants for the access to healthcare resources. Our scoping review is deliberately designed to explore and elucidate the unique landscape of hemophilia care in Brazil. The primary objective is to gain a comprehensive understanding of the national context, encompassing epidemiological trends, burden of the disease, patient journey and unmet needs of the PwH. This focused approach allows us to identify specific areas of concern and potential opportunities for improvement in research, education and healthcare management tailored to the Brazilian setting. By focusing our review specifically on Brazil from the outset, we aim to provide readers with a clear perspective of the existing gaps and particularities in hemophilia care in the national context, which is a crucial aspect in our research outcomes.
MethodsThis study was conducted following the JBI methodology for scoping reviews,6 following a protocol defined a priori7. The report was structured in compliance with The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews (Appendix I).
Search strategy and evidence selectionThe search was performed in MEDLINE (via PubMed), LILACS and the Digital Library of Theses and Dissertations (Biblioteca Digital Brasileira de Teses e Dissertações - BDTD) on July 13, 2022. The search strategy included terms related to the disease (HA and HB) and the context (Brazil), with no restrictions, such as language or date of publication (Appendix II).
The process of study selection was conducted by two independent reviewers at both stages and discrepancies were solved through consensus or by a third reviewer. Whenever a thesis had published scientific papers, preference was given to the selection and extraction of data from the paper. The reasons for exclusions of the full texts have been documented in Appendix III.
This review included quantitative and qualitative studies that presented results of primary or secondary data on the PwH in Brazil related to at least one of the nine research questions stated below. No restriction criterium was applied regarding the study design. Studies published prior to 2002 were not included, as they would not represent the most recent evidence. Studies published in languages other than Portuguese, Spanish or English were excluded.
The research questions regarding HA and HB in Brazil included three concepts:
- 1)
Clinical-epidemiological profile:
- 1.1.
What is the prevalence and incidence of hemophilia in Brazil?
- 1.2.
What are the complications of hemophilia and the complications of its treatment and their respective frequency?
- 1.3.
What is the development frequency of neutralizing antibodies?
- 1.1.
- 2)
Burden of the disease:
- 2.1.
What is the economic impact of hemophilia in Brazil, considering costs for the patient and the health systems (public and private)?
- 2.2.
What is the social impact, emotional impact and impact on the quality of life (QoL) of the PwH in Brazil?
- 2.3.
What are the main outcomes of interest in hemophilia, from the perspective of patients and healthcare managers?
- 2.1.
- 3)
Patient journey and unmet needs:
- 3.1.
What is the adherence rate to the available therapeutic alternatives?
- 3.2.
What is the proportion of patients in the Home Dose Program (HDP)?
- 3.3.
What are the needs and barriers to promoting comprehensive and equal care in the treatment of hemophilia in Brazil?
- 3.1.
Two independent reviewers extracted data for each study, referring to study characteristics, participants, concept (including results) and context. Discrepancies were solved through consensus or by a third reviewer. Relevant data was extracted from studies corresponding with the proposed review objectives (Appendix IV).
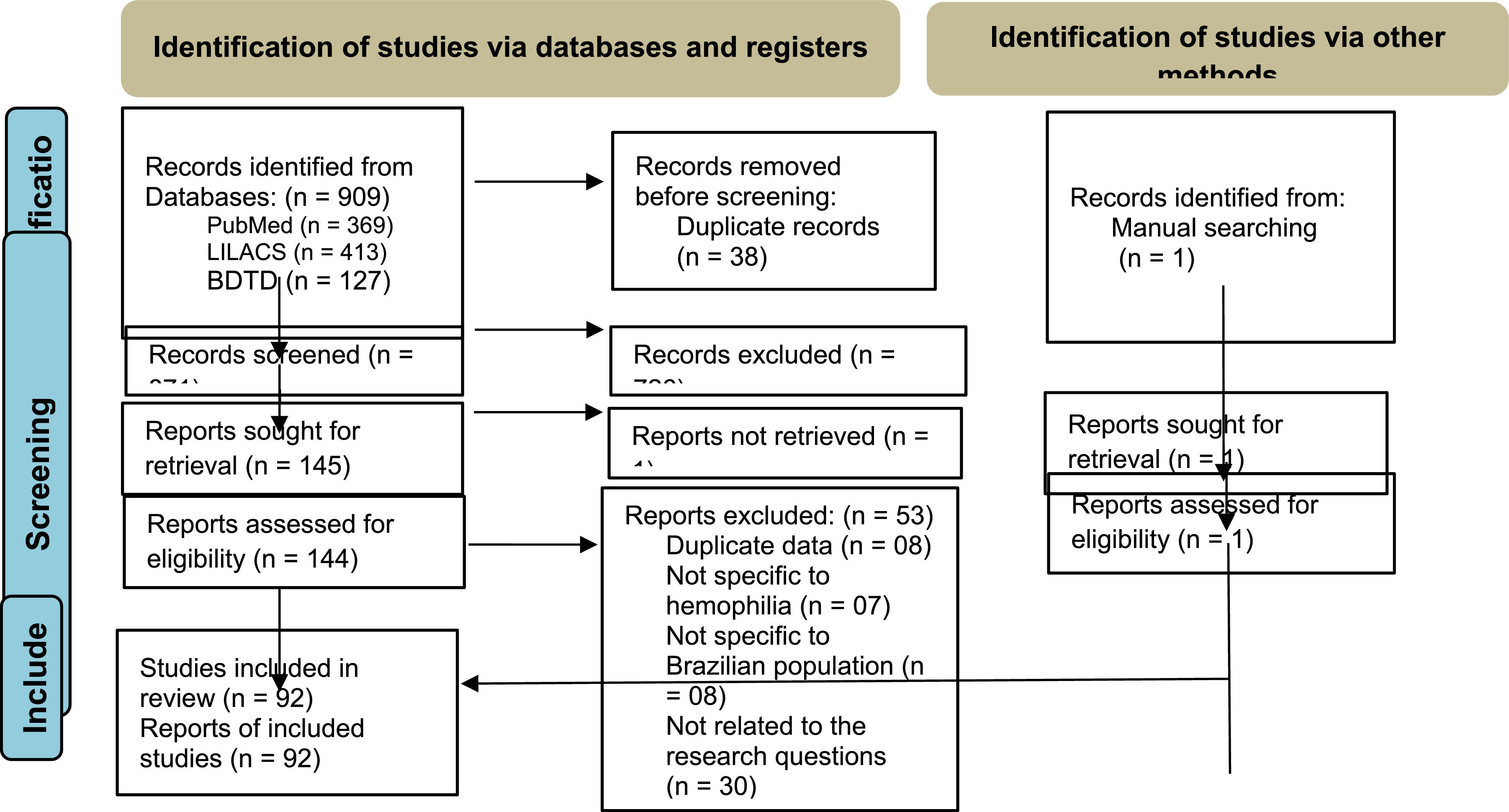
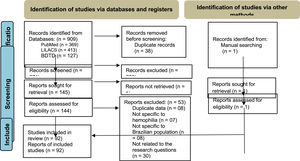
ResultsWe identified 909 references; 145 of these were selected according to titles and abstracts. After the full-text review, 92 references (25 thesis and 67 papers) met the inclusion criteria. The PRISMA flow diagram (Figure 1) outlines the screening and selection process of these articles.
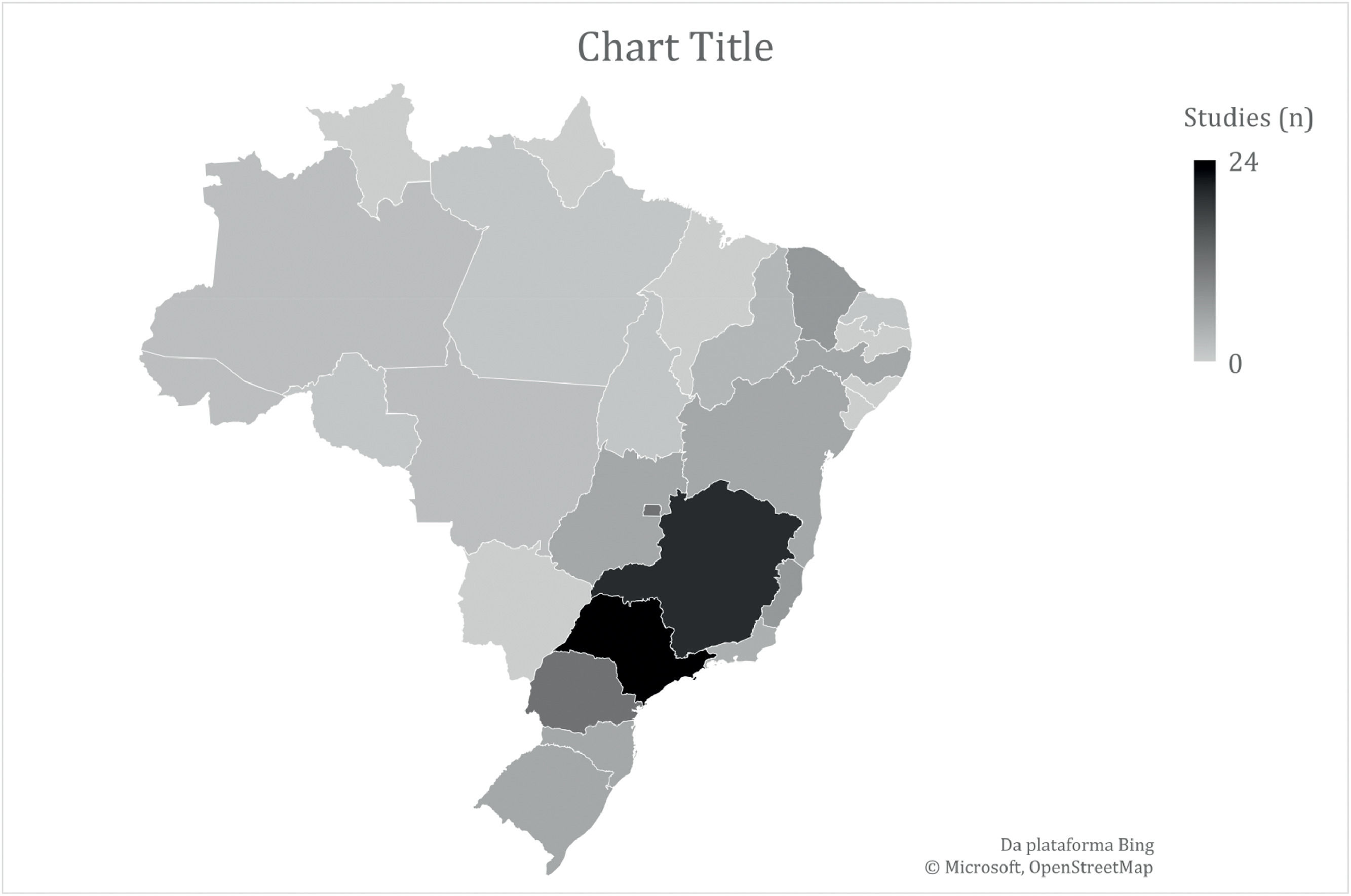
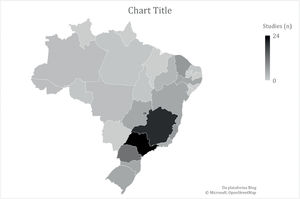
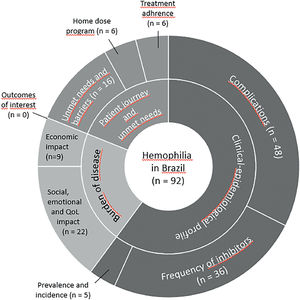
Most studies (n = 60) included populations with HA and HB, six did not specify the hemophilia subtype and 15 included only the HA population. Regarding the context, most studies (n = 76) were in reference to the public healthcare system. Domiciliary care and schools were the setting of interest in one and two studies, respectively, while the context was not informed in 13 studies. Most studies were performed in southeastern and southern Brazil (Figure 2). The number of studies regarding the nine questions is described in Figure 3.
The main findings are reported in Tables 1, 2 and 3 and described according to each concept in this section.
Main findings regarding the research questions of the clinical-epidemiological profile.
| Clinical epidemiological profile | Number of studies | Number of participants (range) | Main findings |
|---|---|---|---|
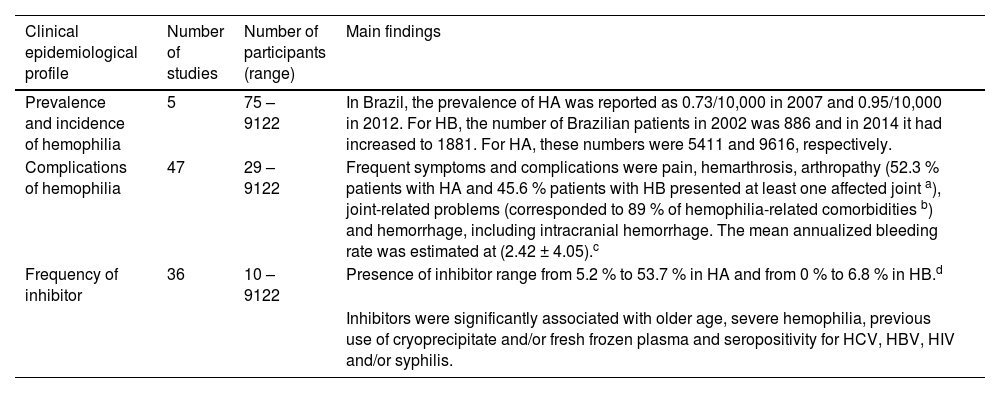
| Prevalence and incidence of hemophilia | 5 | 75 – 9122 | In Brazil, the prevalence of HA was reported as 0.73/10,000 in 2007 and 0.95/10,000 in 2012. For HB, the number of Brazilian patients in 2002 was 886 and in 2014 it had increased to 1881. For HA, these numbers were 5411 and 9616, respectively. |
| Complications of hemophilia | 47 | 29 – 9122 | Frequent symptoms and complications were pain, hemarthrosis, arthropathy (52.3 % patients with HA and 45.6 % patients with HB presented at least one affected joint a), joint-related problems (corresponded to 89 % of hemophilia-related comorbidities b) and hemorrhage, including intracranial hemorrhage. The mean annualized bleeding rate was estimated at (2.42 ± 4.05).c |
| Frequency of inhibitor | 36 | 10 – 9122 | Presence of inhibitor range from 5.2 % to 53.7 % in HA and from 0 % to 6.8 % in HB.d |
| Inhibitors were significantly associated with older age, severe hemophilia, previous use of cryoprecipitate and/or fresh frozen plasma and seropositivity for HCV, HBV, HIV and/or syphilis. |
HA: hemophilia A; HB: hemophilia B.
Main findings regarding the research questions of the burden of the disease.
BRL: Brazilian currency reais. HIV: human immunodeficiency virus; HCV: hepatitis C virus; ITI: Immune tolerance induction; QoL: quality of life; PwH: people with hemophilia.
Main findings regarding the research questions of patient journey and unmet needs.
| Patient journey and unmet needs | Number of studies | Number of participants (range) | Main findings |
|---|---|---|---|
| Adherence to the available therapeutic alternatives | 06 | 27 - 138 | The adherence ranged from 25 % to 72 %. Studies reported adherence by questionnaires of self-perceived degree of adherence and the objective quantification of CFC consumption. Adherence to the treatment seemed to be associated with a younger age, receiving primary prophylaxis, presenting greater use of the recombinant factor and receiving treatment in a hemophilia treatment center. |
| Proportion of patients in a home-dose program | 05 | 17 – 9122 | The proportion ranged from 33 %a to 74 %.b |
| Needs and barriers to promoting comprehensive and equal care in the treatment of hemophilia | 16 | 07 – 9122 | Distance from home to the outpatient clinic, lack of coordination of specialized and emergency care for the PwH, insufficient training of healthcare professionals on hemophilia; lack of education on hemophilia for the PwH, restricted access to physiotherapy and rehabilitation. |
CFC: clotting factor concentrate; PwH: people with hemophilia.
A total of 66 studies (52 papers and 14 thesis) addressed the concept “Clinical-epidemiological profile”, many of them with results informing more than one research question (Tables 1 and Appendix IV). Five studies investigated the presence of the PwH in the country or in specific regions, most of them using results from the web-based national registry of hemophilia - the Hemovida Web Coagulopatias. In Brazil, a prevalence of 0.95/10,000 in 2012 for HA and the absolute number of 1881 PwH B in 2014 were identified. It is possible that few studies on this topic are observed, as this data is only available in the national registry Hemovida Web, which was implemented in 2009 and includes all Brazilian patients with inherited bleeding disorders treated in the public sector, corresponding to approximately 100 % of patients with moderate/severe forms.13 The Ministry of Health periodically publishes an official document to publicize the information registered in the Hemovida Web, including the prevalence of hemophilia.14
Symptoms, comorbidities and/or complications related to hemophilia or related to hemophilia treatment were investigated by 48 studies. Most studies reported complications of hemophilia treatment, as seroprevalence of infections of HIV, HCV and HBV, ranging from 0 % to 23.4 %, 14.3 % to 63.2 % and 1.9 % to 49 %, respectively. Frequent complications of the disease finding included hemarthrosis, arthropathy, joint related problems (with greater impairment in the knees, elbows and ankles) and hemorrhage, whereas the last one has been cited as a main cause of death. A multi-national cohort study reported the mean annualized bleeding rate in Brazil at 2.42 ± 4.05.10
Related to the last question of this concept, the presence of inhibitors was reported in 36 studies. Seven of them showed the frequency of inhibitors for the Brazilian population, ranging from 7.3 % to 53.7 % in HA and from 1.4 % to 1.9 % in HB. Considering studies with populations from a specific city or state, inhibitor frequency has a slight difference, ranging from 5.2 % to 31.5 % in HA, and from 0 % to 6.8 % in HB. The history of inhibitor detection during the lifetime showed that this outcome was significantly associated with older age, severe hemophilia, previous use of cryoprecipitate and/or fresh frozen plasma and seropositivity for HCV, HBV, HIV and/or syphilis. Exclusive use of virus-inactivated clotting factors was associated with a decreased chance of developing inhibitors.15
Burden of the diseaseThirty-one studies (21 papers and 10 theses) investigated the concept of “Burden of disease” (Table 2 and Appendix IV). Nine studies (5 papers and 4 theses) investigated the economic impact of hemophilia in Brazil. In 2003, the Ministry of Health adopted a new system for the acquisition of CFCs, which lowered the price by about 36 %.16 However, in the first three years since the implementation of the prophylactic treatment in the Brazilian Public Health System in 2011, the direct costs of treating hemophilia increased 286.8 %.17 During the period from 2011 to 2015, the average annual cost per patient was BRL 57,416.43 (BRL 2962.10 in mild hemophilia and R$ 89,467.77 in severe cases), with no significant difference between HA and HB. Blood products corresponded to 99.46 % of the total cost.17 In 2018 to 2019, the plasma Factor VIII cost BRL$ 1569.75 per patient/month, while the original patent recombinant Factor VIII (Moroctocog alpha) cost BRL$ 9993.70 per patient/month and the recombinant Factor VIII produced by the Brazilian Company of Blood Products and Biotechnology (Empresa Brasileira de Hemoderivados e Biotecnologia - Hemobrás) cost BRL$ 5606.25 per patient/month. As for Factor IX in plasma, the cost was BRL$ 1142.00 per patient/month. The original patent Recombinant Factor (Eftrenonacog alpha) cost BRL$ 7440.00 per patient/month, but this technology was not provided by Hemobrás.18 In this context, the recombinant Factor IX is one of the most requested medications through legal injunctions,18–20 being responsible for 22.53 % of the costs of judicialized drugs.17 For the PwH with inhibitors, the cost of the ITI was significantly lower (USD$ 101,943.5; range 3938 to 894,675.6), when compared to the cost of the treatment in the 12 months prior to the ITI for the PwH with a completely successful ITI (USD$ 173,521.45; range 0 to 1291,244.2; p = 0.035). The treatment of the PwH achieving partial success showed a significantly higher cost during the ITI period (USD$ 389,521.00; range 112,233 to 842,581.65) because some patients needed to start secondary prophylaxis with bypassing agents along with the ITI.21 The bypass agent recombinant activated factor VII (rFVIIa) costs about 8 % to 43.7 % less than the activated prothrombin complex concentrate (aPCC).22,23 In 2013, the cost with bypassing agents was BRL$ 180,962,263.00 for the public health system, with hemophilia A and B being responsible for the consumption of 22.1 % to 97 %, according to the type of agent.24
Regarding the intangible costs of hemophilia, 22 studies (16 papers and 6 theses) investigated the social impact, emotional impact and QoL of the PwH in Brazil. The studies used qualitative interviews and quantitative instruments to assess the QoL, some specific for the PwH, such as the Haemophilia-Specific Health-Related QoL Questionnaire for Adults (HAEMO-QoL-A) and the Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool (CHO-KLAT) and some non-disease-specific, such as the SF-36, WHOQOL-BREF, EuroQol-5D (EQ-5D), the child-friendly EQ-5D version (EQ-5D-Y) and the Pediatric QoL Inventory (PedsQoL). The presence of HIV-infection, HCV-infection and target joint impairment were related to the impaired QoL in the PwH.25 In general, the most impaired domains of the QoL of the PwH were physical functioning, pain and school or work impairment. About 43 % to 82.6 % of the PwH declared themselves unemployed and 57.1 % to 71 % did not receive government financial assistance. Thirty-five percent of the PwH present depressive symptoms and 18 % receive treatment for depression or anxiety. Family members and caregivers also reported an impact on their QoL and working life and 29 % declared themselves recipients of treatment for depression or anxiety.
Patient journey and unmet needsA total of 26 studies (19 papers and 7 thesis) addressed the concept “Patient journey and unmet needs” (Table 3 and Appendix IV). Six studies investigated the adherence to the available therapeutic alternatives, for which a range of 25.4 % to 72.5 % of the PwH were considered adherent to the treatment. Patients adherent to the treatment seem to be of a younger age and receiving primary prophylaxis and to present greater use of the recombinant factor.26 Adolescents that showed “poor adherence” to the treatment were over 14 years old and underwent autoinfusion.12 Receiving treatment at a hemophilia treatment center proved to be a positive and independent factor associated with the compliance with the treatment protocol (Odds Ratio: 2.388; 95 %CI: 1.052 – 5.418).27 Regarding the proportion of patients in the Home Dose Program (HDP), six studies showed the participation of 33.3 % to 90 %. The recent survey published in 2016 by the Brazilian Ministry of Health14 reported that 49.81 % and 43.64 % of the PwH A and B, respectively, participate in the HDP.
Finally, 16 studies (11 papers and 5 theses) investigated the needs and barriers to promoting comprehensive and equal care in the treatment of hemophilia in Brazil. The distance from home to the treatment center appeared as a barrier. Difficulties related to transportation and the distance from home to hemophilia treatment services were found to be reasons for nonadherence to treatment,28,29 as patients would often need to move to a closer location.30 In this context, the HDP seems to be a reasonable alternative for the treatment of the PwH, since the reduction in the occurrence of sequels, number of infusions, pain episodes and absences from school or work were considered advantages reported by the PwH and their families.31 Meanwhile, the reported disadvantages were related to possible reactions, lack of confidence in the dosage to be used and, in some cases, sociocultural and financial factors which made it impossible to store and infuse procoagulants at home or enable patients to go to the referral service for the monitoring and administration of regular doses.31,32 Limitations in relation to the structure of outpatient clinics, the organization of care for the PwH, especially in emergency care, physiotherapy and rehabilitation services, restricted management of inhibitors, training of human resources specialized in hemophilia care and existence of few research centers were also identified as barriers to promoting comprehensive care for the PwH.
DiscussionThe current scoping review investigated epidemiological issues, burden of disease, patient journey and unmet needs for the PwH in Brazil. Ninety-two studies were included and addressed all research questions except the Main outcomes of interest in hemophilia, from the perspective of patients and managers in healthcare (question 2.3). The distribution of the Brazilian studies is not even in the country, studies conducted in the states of São Paulo and Minas Gerais are more frequent in the literature.
Although some studies provided data on the prevalence of HA and HB, none of them were developed specifically to investigate the prevalence and incidence of these coagulopathies in Brazil. Therefore, information on this epidemiological aspect comes almost exclusively from the Ministry of Health through the Hemovida Web Coagulopatias system implemented in 2009 or through a periodical report. Despite it being estimated that almost 100 % of the PhW in Brazil are registered the in Hemovida Web Coagulopatias, this system has limitations in identifying patients who may not use the public health system, as well as in identifying duplicate patients, aside from the possible incompleteness of the information.
There are also few studies that specifically aimed to investigate the prevalence of inhibitors or the relationship between the prevalence of inhibitors and other aspects of the disease (severity, complications, QoL or mortality). The findings of the current review on the prevalence of inhibitors are quite divergent, since we found a great heterogeneity in the study designs and population (e.g., severe hemophilia or individuals carrying certain genetic mutations).
Most of the studies found reported the seroprevalence of viral infections, such as HIV, HCV and HBV. Viral infections are the most common complications of the hemophilia treatment, especially in those PwH born before the mid-1980′s, when effective methods of viral inactivation of CFCs were introduced. It has been estimated that after the implementation of viral inactivation methods and screening for viral infection in blood donors, the risk of viral infections resulting from the hemophilia treatment has been reduced in Brazil. However, the current scoping review found no studies on this topic.
Regarding the complications of hemophilia itself, osteoarticular complications and pain emerged in the current review as the most impacting elements on the physical functioning and QoL of the Brazilian PwH. This finding corroborates the current evidence that chronic arthropathy, which is often associated with deformity and instability, flexion contractures, muscle atrophy and pain, is a major determinant of the health-related QoL in the PwH and the general impairment of physical dimensions results in lower levels of QoL,33 not only for those with severe hemophilia, but also for non-severe hemophilia, despite low joint bleeding rates.34 Furthermore, the restricted access to physiotherapy and rehabilitation was found as an unmet need for the PwH in Brazil. Data on the number of days lost from work/school each year and its association with the physical functioning limitation, as well as with osteoarticular complications, have not yet been studied in the Brazilian population. Moreover, the investigation of the employment status of the PwH is restricted.
The latest advances in hemophilia treatment led to a change in the aim of treatment from increasing life expectancy to preventing joint damage and protecting against joint bleeding and hemophilic arthropathy.35 Therefore, adherence to the treatment is important in the maintenance of the health status. However, logistical difficulties in reaching the hemophilia treatment center were raised as the main barrier of the PwH in Brazil and adherence to the prophylactic treatment rates were shown to vary from 25 % to 72 %, which is in line with results obtained in a previous study applying analysis for the global and US PwH populations.36 In this context, the HDP seems a promising strategy to overcome logistical barriers and to reduce abstention from work/school. However, the Brazilian PwH may feel a lack of confidence in the dosage to be used, fear of possible reactions or may face difficulties in storing the medication at home.31,32 Controversially, receiving treatment at a hemophilia treatment center proved to be a positive factor associated with the adherence to the treatment27 and adolescents who underwent auto-infusion presented poor adherence to the treatment.12 Still, research on the factors associated with adherence to home and outpatient treatment is necessary to improve the screening of candidates for each type of treatment setting.
Although no study has addressed the main outcomes of interest in hemophilia, from the perspective of patients and managers in healthcare, a report from the Ministry of Health14 mentions the need for epidemiological surveillance of inhibitors, infections, osteoarticular complications and chronic diseases and mortality. This report also mentions those records in the Hemovida Web Coagulopatias system as fundamental in the correct monitoring of this population, giving it a longer life, and the initiation of the presentation of chronic degenerative diseases that are characteristic of the general population, encouraging the implementation of policies aimed at improving the quality of care provided to patients.
To our knowledge, this is the first scoping review aiming to map the Brazilian publications on hemophilia referring to these three concepts: (i) clinical-epidemiological profile; (ii) burden of the disease, and; (iii) patient journey and unmet needs. As a limitation, the great heterogeneity of study designs and samples hinders the interpretation of data, a common issue observed in scoping reviews of clinical conditions. Still, the aim of this scoping review was to investigate and gather what is known about HA and HB in Brazil and guide future research and support for the implementation of health policies for the PwH and not to answer the framed questions.
ConclusionThe evidence provided by this scoping review shows results for the three concepts established, the clinical-epidemiological profile, burden of the disease and patient journey and unmet needs. Hemophilia still poses a considerable burden on the PwH, despite the available modalities of treatment. There are remaining medical and social unmet needs that should be addressed by researchers and policymakers in the future, such as the low rates of adherence, disease complications, and unemployment. Only one question related to main outcomes of interest in hemophilia could not be answered. This exposes a gap in the literature and offers opportunities for future studies.
This study was sponsored by Pfizer.