Inherited hemophilia A is a rare bleeding disorder due to absent/reduced activity of the clotting factor VIII (FVIII).1 Therefore, its treatment is based on regular FVIII infusions to prevent (i.e., prophylaxis) or treat (i.e., on demand) bleedings.1 The most impacting complication of this treatment is the development of anti-FVIII antibodies (i.e., inhibitors).1 Inhibitors reduce/abolish the clotting activity of FVIII, rendering the patients with the previous or worse bleeding phenotype, which ultimately leads to increased morbimortality.1 Although bypassing agents (recombinant activated factor VII [rFVIIa] and partially activated prothrombin complex [aPCC]) can be prescribed for both prophylactic and on-demand treatments, the current recommended prophylactic drug in such cases is the humanized bispecific antibody emicizumab.1,2 Emicizumab mimics the clotting effect of FVIII and, since its structure is not similar to the FVIII structure, anti-FVIII inhibitors have no effect on its clotting activity.1,2 There are few reports about the hemostatic effectiveness and safety of this biopharmaceutical on major non-orthopedic surgeries.3–5 In addition, since the introduction of emicizumab in the armamentarium for hemophilia treatment in Brazil, in 2018, no major surgical procedures have been reported in Brazilian patients on emicizumab prophylaxis. Herein we described a man with severe hemophilia A and high-responding inhibitor under emicizumab prophylaxis who was submitted to open cholecystectomy due to acute-on-chronic calculous cholecystitis. This report was approved by the local Committee on Ethics on Research (May/29/2019). The patient signed the Consent Form before his data was reviewed on the hospital medical files.
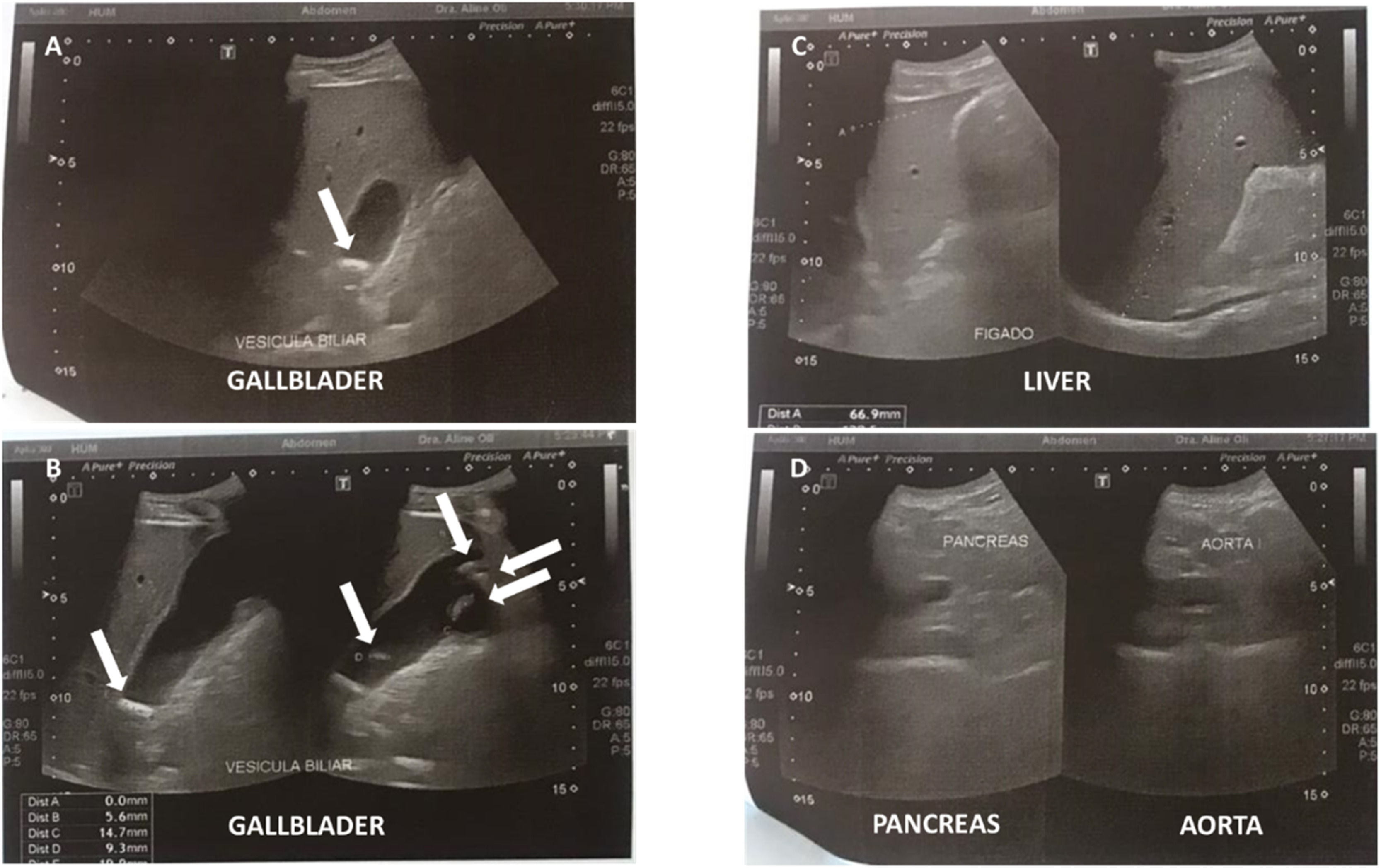
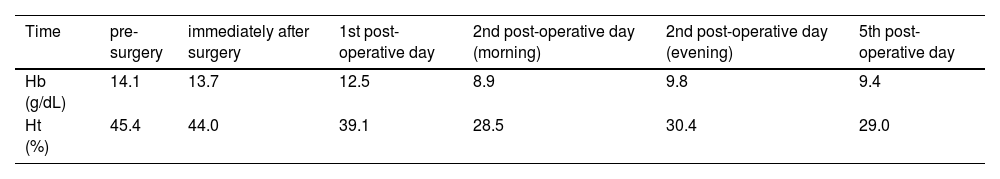
Case reportA 32-year-old man first visited the Emergency Department complaining severe diffuse abdominal colic unresponsive to oral analgesics, in the last 7 h. He denied fever, nausea, and diarrhea. He had severe hemophilia A with high-responding inhibitor. He was under prophylaxis with emicizumab (1.5 mg/kg weekly). He had been submitted to several abdominal surgeries (appendicectomy, midgut volvulus, and abdominal trauma). Physical examination was unremarkable. Aspartate and alanine aminotransferases were mildly elevated (56 and 83 U/L, respectively). He was discharged home after ameliorating with symptomatic drugs. He came back 4 days later, with the same complaints. He had a positive Murphy's sign and a negative Blumberg's sign. Blood tests were unremarkable. The gallbladder had a normal wall thickness (0.6 cm) with several mobile calculi (from 0.9 to 1.8 cm) by abdominal ultrasound, without dilation of the biliary tract, alteration of the pancreas/pancreatic duct, or lymphadenomegaly (Figure 1). He declined hospitalization for probable surgical assessment, but he returned 5 days later, after having discussed with his Hematologist. Emicizumab was maintained throughout the described period. Before the procedure, he received intravenous rFVIIa 93 µg/kg and tranexamic acid (TxA) 1 g. rFVIIa was maintained each 2 h, during the first 24 h. The procedure was performed under general anesthesia. Videolaparoscopic cholecystectomy was withheld, due to adhesions precluding peritoneal inflation. Upon a right subcostal (Kocher) incision, a hydropic gallbladder was removed. Anatomopathological analysis confirmed acute-on-chronic calculous cholecystitis. A purified pigskin sterile absorbable gelatin sponge was placed on his hepatic bed, before closing the surgical wound. The surgeon reported above-the-usual bleeding during the procedure (estimated blood loss of 500 mL against usually minimal blood loss). Ceftriaxone and metronidazole were prescribed throughout the hospitalization. rFVIIa 77 µg/kg was scattered daily: every 2 h until every 12 h along 1 week (total 2400 µg/kg). TxA 1 g every 8 h was prescribed during the same period. He had mild hematic drainage (less than 30 mL on the post-operatory day and decreasing in the following days). This was judged as good hemostasis by the surgeon. The drain was removed on the 4th post-operatory day. Hemoglobin decreased from 13.7 g/dL on the day of the procedure to 9.4 g/dL the day before discharge (lowest 8.9 g/dL) (Table 1). No blood transfusion was required throughout the hospitalization. He was discharged home 1 week after surgery. No bleeding event was reported during the outpatient follow-up within 3 weeks. No symptoms nor signs of thrombosis were reported.
Abdominal ultrasound performed on the 5th day of symptoms. (A and B) Several mobile calculi (from 0.9 to 1.8 cm) were identified inside the gallbladder. White arrows point the calculi. (C) Both liver echotexture and intra- and extra-hepatic canaliculi were normal. (D) Pancreas echotexture was normal.
To the best of our knowledge, this is the first report of a Brazilian man with severe hemophilia A and high-responding inhibitor under prophylactic emicizumab who was submitted to major non-orthopedic surgery. Worldwide, the experience on major surgical procedures on people with hemophilia A under emicizumab prophylaxis largely involves orthopedic surgeries,3–5 which are the most prevalent surgical procedures to which these patients are submitted.1 There are few reports on major non-orthopedic surgeries in such cases. In the pivotal studies of efficacy and safety of emicizumab prophylaxis, among 18 major surgical procedures, five were non-orthopedic, of which one was cholecystectomy.3 Among inhibitor-positive patients, the authors reported that the hemostasis provided by the concomitant use of emicizumab and bypassing agent was good.3
While emicizumab is a prophylactic agent, hemostasis to treat bleeding among inhibitor-positive individuals should be accomplished with bypassing agents.1 aPCC is not recommended as first-line therapy due to the risk of thrombosis when used concomitantly with emicizumab.1 However, concomitant use of rFVIIa and emicizumab has a safe profile and there are recommendations for their prescription during major orthopedic surgeries.1,6,7 For major orthopedic surgeries, an expert group recommended rFVIIa 90–120 µg/kg and TxA pre-operatively and de-escalating rFVIIa frequency every 2 days, based on published evidence and personal experience.7 For non-orthopedic surgery hemostasis without emicizumab coverage, some experts advocate a bolus dosing of 120 µg/kg and maintenance dose of 90–120 µg/kg every 2 h, de-escalating daily by increasing the time between infusions, until there is no sign of bleeding and the wound is normally healing.8 We opted to use the lowest-dose regimen since the patient was under emicizumab prophylaxis. Although there was a minimal bleed and a significative drop on the patients’ hematimetry, no transfusion was required.
The incorporation of emicizumab as a prophylactic therapeutic for people with hemophilia A and high-responding inhibitor resulted in an effective reduction of breakthrough bleeding as well as less hemorrhage after low-to-moderate trauma.2 However, its effectiveness as a hemostatic agent in overall major surgeries remains to be elucidated.3 Finally, we would like to reinforce the importance of an interdisciplinary approach as the best option to manage surgery among people with hemophilia, despite severity, inhibitor status, and product in use. In conclusion, the concomitant use of emicizumab and rFVIIa was effective and safe for a man with severe hemophilia A and high-responding inhibitor submitted to open cholecystectomy.
Authors’ contributionsP.C.G. was responsible for the conceptualization, data curation, investigation, and writing – review & editing. M.T.B. was responsible for the investigation and writing – review & editing. J.A.-T. was responsible for the conceptualization, formal analysis, methodology, project administration, supervision, visualization, writing – original draft, and writing – review & editing. R.M.C. was responsible for the conceptualization, data curation, formal analysis, methodology, project administration, supervision, visualization, writing – original draft, and writing – review & editing.
FundingThis report did not receive any funding or grants to be planned, performed, or published.
None.