Overall, mature B-cell neoplasms with more than one malignant cell population represents less than 5% of B-cell diseases.1 Though a well-recognized phenomenon, the clinical implications of some combinations of mature B-cell neoplasms have not been determined yet.
Hence, with the goal to draw attention to this fact, while also emphasizing the role of a detailed blood smear analysis as a tool for indicating the presence of two or more distinct cell populations, here we describe a patient with concomitant monoclonal B-cell lymphocytosis (MBL) and CD5−/CD10− mature B-cell neoplasm.
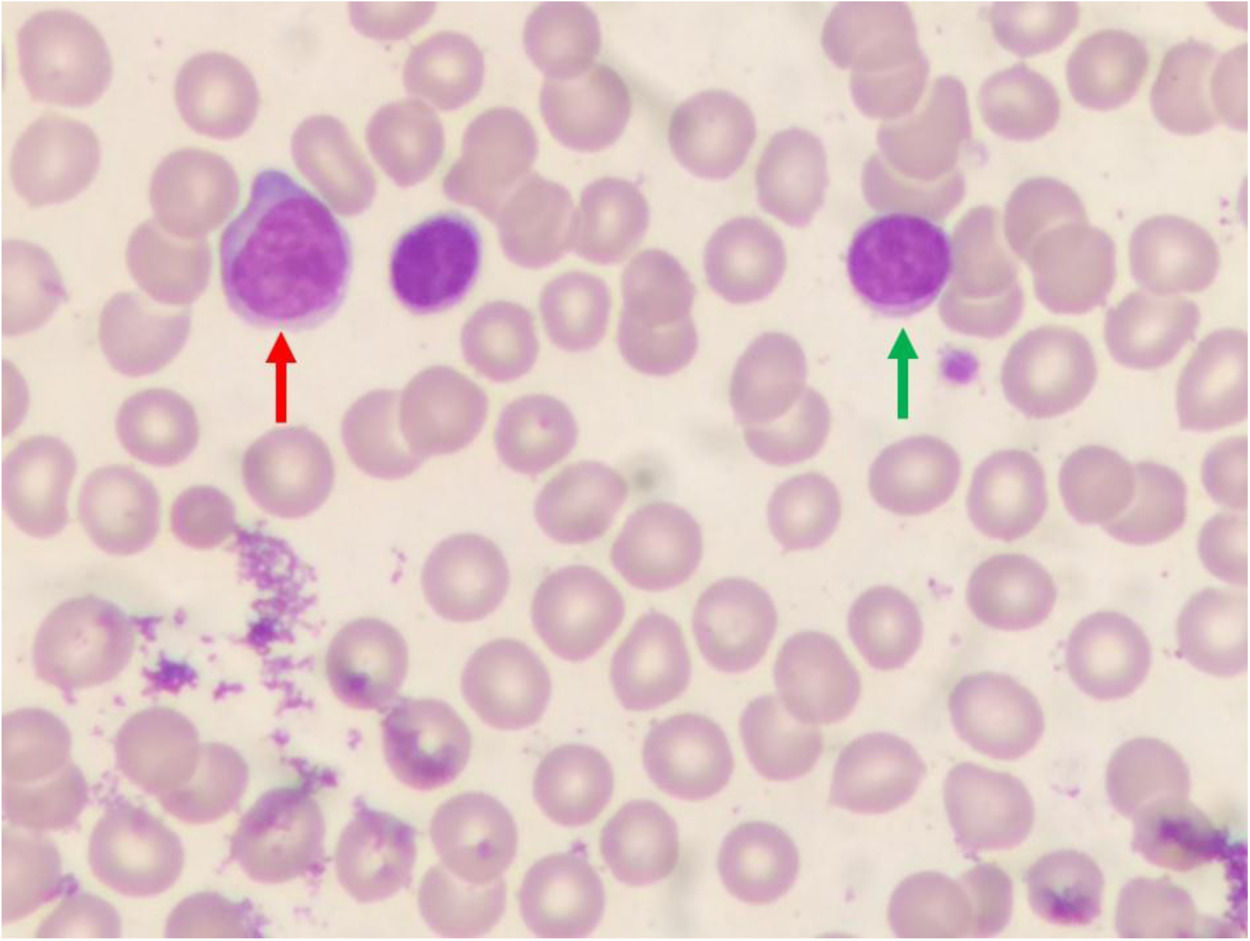
Case reportA 65-year-old man was referred to our laboratory with a request for an immunophenotyping of peripheral blood. Unfortunately, we did not receive any clinically relevant information, except that the patient was asymptomatic. The blood smear revealed two different populations of lymphoid cells: a small one, characterized by a nucleus with clumped and homogeneous chromatin, and a scanty cytoplasm; and a larger one, whose nucleus showed more dispersed chromatin, and more abundant blue-pale cytoplasm (Figure 1).
For the immunophenotypic analysis by flow cytometry, the following panel of antibodies (clones in brackets) was used: CD2 FITC (RPA-2.10), CD3 PerCP-Cy5.5 (UCHT1), CD4 PE (L200), CD5 APC (UCHT2), CD8 FITC (SK1), CD10 FITC (J5), CD11c PE (3.9), CD19 PerCP-Cy5.5 (HIB19), CD20 FITC (2H7), CD22 FITC (S-HCL1), CD23 PE (EBVCS-5), CD24 PE (ML5), CD25 PE (M-A25), CD34 PE (8G12), CD38 APC (HIT2), CD43 FITC (1G10), CD45 APC (HI30), CD58 FITC (1C3), CD79b PE (CB3-1), CD81 FITC (JS-81), CD103 FITC (Ber-ACT8), CD123 PerCP-Cy5.5 (7G3), CD200 PE (MRC OX-104), FMC7 FITC (FMC7), HLA-DR FITC (L243), IgM FITC (F0058), kappa FITC (G20-193), kappa PE (R0436), lambda FITC (F0435), and lambda PE (JDC-12). A total of 50.000 events was acquired per tube.
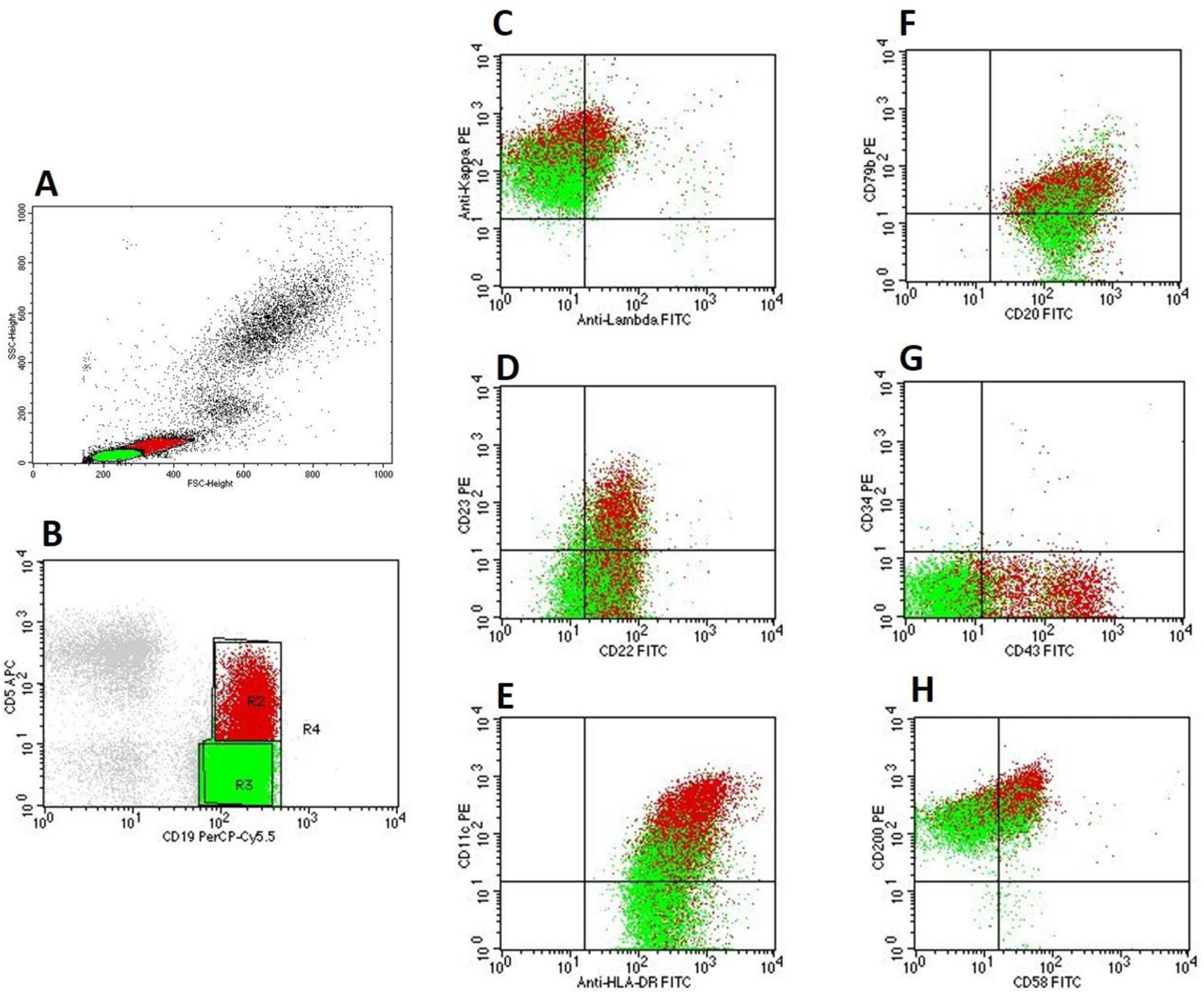
The immunophenotyping corroborated the presence of two distinct cell populations which were visualized in the blood smear: ‘green’ (FSCvery low, SSCvery low) and ‘red’ (FSCintermediate, SSClow) (Figure 2A). Both populations were negative for CD2, CD3, CD4, CD8, CD10, CD34, CD103, CD123, and FMC7.
Flow cytometry findings of the patient's blood. A: Flow cytometry's physical parameters (FSC and SCC) show two different populations of lymphoid cells: ‘green’ (FSCvery low, SSCvery low) and ‘red’ (FSCintermediate, low SSClow). B: ‘Green’ B-cells: CD5−/CD19+ and ‘red’ B-cells: CD5+/CD19+, C. Kappa light-chain restriction of ‘green’ (kappalow) and ‘red’ (kappamoderate) B-cells. D, E, F, G, H: ‘Green’ B-cells were CD11c+, CD20moderate, CD22low, CD23−, CD34−, CD43−, CD58partial, CD79blow, CD200moderate, and HLA-DR+; ‘red’ B-cells were CD11c+, CD20moderate, CD22low, CD23+, CD34−, CD43+, CD58+, CD79blow, CD200bright, and HLA-DR+.
The ‘green’ population was characterized by CD5−, CD19+, and kappalow light-chain restriction (Figure 2B, C). It was also CD11c+, CD20moderate, CD22low, CD23−, CD43−, CD58partial, CD79blow CD200moderate, and HLA-DR+ (Figure 2 D, E, F, G, H), and also CD24+, CD25partial, CD38+, CD45bright, CD81+, and IgM+ (data not shown). The absolute B-cell count was 6.3 × 109/L. The diagnosis of CD5−/CD10− mature B-cell neoplasm was made. Based on the cytomorphological and immunophenotypic findings, it was not possible to exclude the diagnosis of lymphoplasmacytic lymphoma or marginal zone lymphomas.2 However, there were not enough subsidies to determine a specific diagnosis.
On the other hand, the ‘red’ population was CD5+, CD19+, and kappamoderate light-chain restriction (Figure 2B, C). It was also CD11c+, CD20moderate, CD22low, CD23+, CD43+, CD58+, CD79blow, CD200bright, and HLA-DR+ (Figure 2 D, E, F, G, H), and also CD24+, CD25+, CD38+, CD45bright, CD81+, IgM+ (data not shown). The absolute B-cell count was 2.7 × 109/L, which is characteristic of CLL/SLL-type monoclonal B-cell lymphocytosis (MBL) (Matutes’ score 4).2
Significantly, there is a possibility that this case could be an example of a CD5−/CD10− B-cell neoplasm with a ‘low burden’ CLL-like subclone, instead of a concomitant CD5−/CD10− mature B-cell neoplasm and CLL/SLL-type MBL.1 To prove real biclonality, it would be important to show that the two populations described were cytogenetically and/or molecularly unrelated, which could be accomplished by the use of FISH and/or PCR for immunoglobulin heavy chain gene (IGHV) rearrangements, respectively.1 Unfortunately, those tests were not done. However, it is important to take in account that there are some biclonal B-cell diseases which show only one IGHV subtype.3 Ultimately, it seems to us that the presence of two cytomorphologically different populations of lymphoid cells with such a distinct immunophenotype when compared to each other, points to two unrelated mature B-cell neoplasms in co-existence.
CommentsThis case highlights the importance of a detailed review of the cytomorphological aspects of the blood smear as a guide for the immunophenotypic study. In fact, the presence of two cytomorphologically distinct types of lymphoid cells was suggestive of more than one immunophenotypically aberrant population.
Furthermore, concerning CLL-like MBL patients with more than one B-cell clone, the incidence was found to be approximately 13% in low-count MBL and 23% in CLL/SLL-type MBL.4 As we have previously shown, while low-count MBL is probably just a ‘sign’ of immune senescence − being the progression of low-count MBL to CLL/SLL-type MBL and CLL extremely rare −5 CLL/SLL-type MBL evolves to CLL requiring therapy at a rate of 1% to 2% per year.6 Substantially, this can be the result of several immune imbalances associated with and/or caused by CLL/SLL-type MBL, as those seen in B-cells,7 T-cells,7,8 chromosomes,9 genes,10 micro-RNAs,11 and telomere length.12
Thus, what is the clinical significance of the concomitant association of CLL/SLL-type MBL and mature B-cell neoplasms? The simple and direct answer is that, at present, we simply do not know. However, given that preliminary data show that a higher percentage of CLL cases requiring early treatment was found among patients with a CLL clone associated with non-CLL clone (as compared with monoclonal CLL patients),1,3 it is reasonable to ask if CLL/SLL-type MBL patients with concomitant mature B-cell neoplasms, as we found in the present case, are more prone to progression to CLL requiring therapy than single-clone MBL patients.
As other problematic questions related to MBL (as, for example, the role of prognostic factors and the implications of MBL in transplant donors), the issue concerning the co-presence of CLL/SLL-type MBL and mature B-cell neoplasms should be included in the roll of unresolved queries of MBL.
Author contributionD.M.M performed flow cytometry analysis, reviewed the literature, and wrote the manuscript.
Ethics statementNot applicable: single case report.
This case was diagnosed while I was the medical scientific advisor and the coordinator of the Flow Cytometry Section of the Clementino Fraga Laboratory (Fortaleza, CE, Brazil). For all the support, I am grateful to that institution.