Acute lymphoblastic leukemia (ALL) is characterized by proliferation of blasts that are committed to B cell or T cell lineage.1 A particular challenging subtype is early T cell precursor (ETP)-ALL. ETP-ALL has unique features compared with other subtypes of T-ALL, and has attracted attention due to its refractoriness to chemotherapy.2
The immunophenotype of ETP-ALL is unique and characteristic. Blasts are positive for CD7, CD2, and cytoplasmic CD3. They are also positive for myeloid-associated antigens, such as CD34, CD117, CD13, CD11b and HLA-DR, whereas myeloperoxidase (MPO) is negative and CD4 may be positive in some cases.3,4 On the other hand, CD5 is negative/weakly positive (expressed in up to 75% of blasts).5 Of note, CD1a and CD8 are typically negative on ETP-ALL blasts.
The Abelson leukemia viral oncogene (ABL1), located at 9q34, is a proto-oncogene with tyrosine kinase activity. Its constitutive activation can occur via a BCR-ABL1 fusion gene generated by t(9;22). The phenomenon of ABL1 gene deletion without BCR-ABL1 rearrangement in ALL patients is rarely reported.1 Up to now, only six cases of ABL1 deletion have been reported in previous literature and all of these cases were reported in B cell ALL. Here, we discuss the first case of ETP-ALL that shows ABL1 deletion, without BCR-ABL1 rearrangement, detected by fluorescence in situ hybridization (FISH).
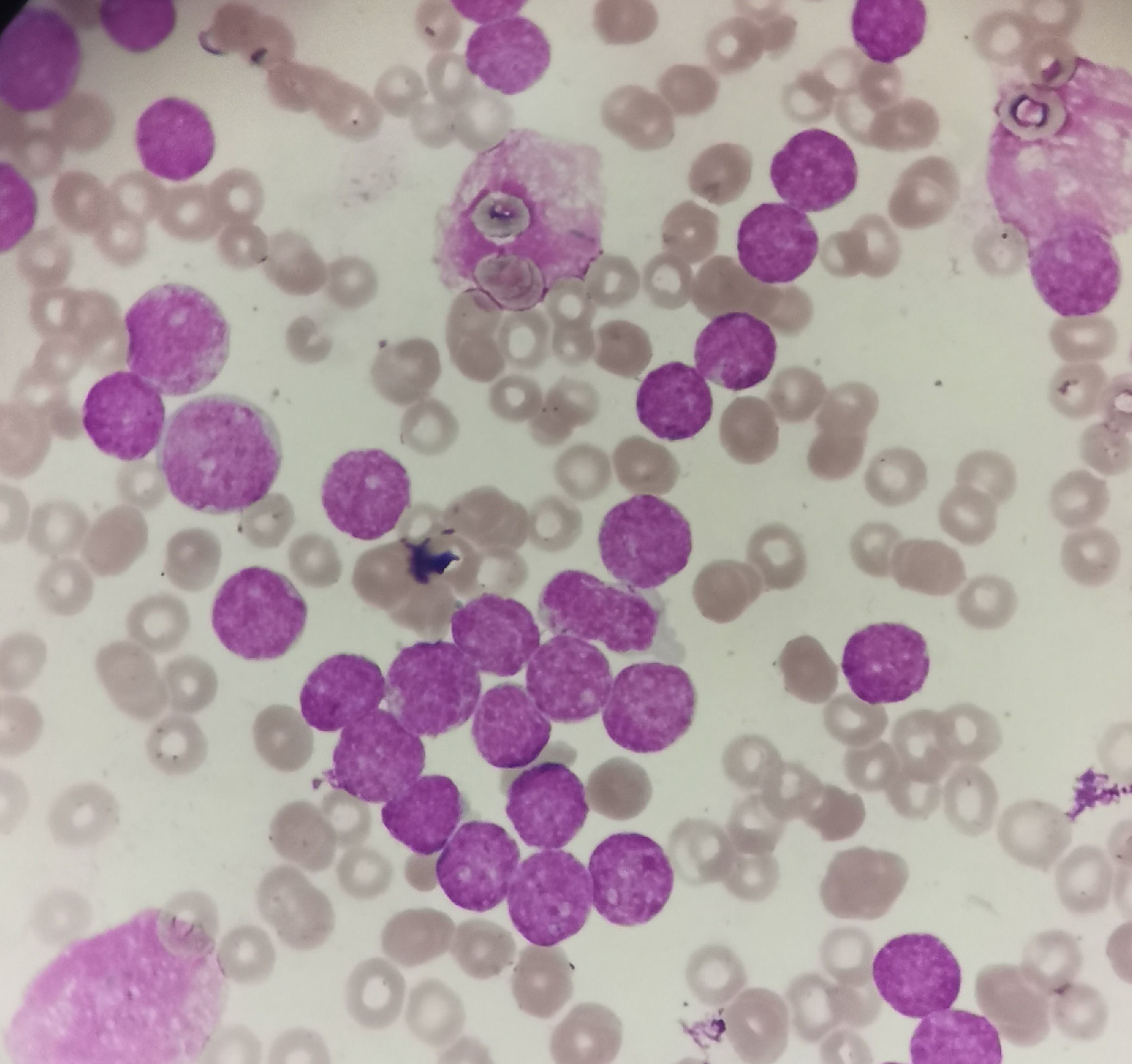
Case reportA 4-year-old male child presented with fatigue and pallor for 2 weeks. The patient had no organomegaly and lymphadenopathy. Initial complete blood count showed a hemoglobin level of 6.3 g/dL, a platelet count of 11 × 109/L and a white blood cell count of 324 × 109/L with presence of 95% blasts (Figure 1). Bone marrow (BM) aspiration revealed 98% blasts. Flow cytometric immunophenotyping revealed that the blasts (CD45 dim) were positive for cytoplasmic CD3, CD34, CD33, CD2, CD7, HLA-DR and CD117. They were negative for CD4, CD8, CD1a and cytoplasmic MPO. CD5 is weekly positive. These findings diagnosed the case as ETP-ALL.
Cytogenetic testing was performed using ALL FISH panel that includes BCR-ABL1, KMT2A-AFF1, TEL-AML1, E2A-PBX1 dual fusion probes, MYC rearrangement and IGH rearrangement break apart probes (Cytocell Ltd, Oxford Gene Technology, UK).
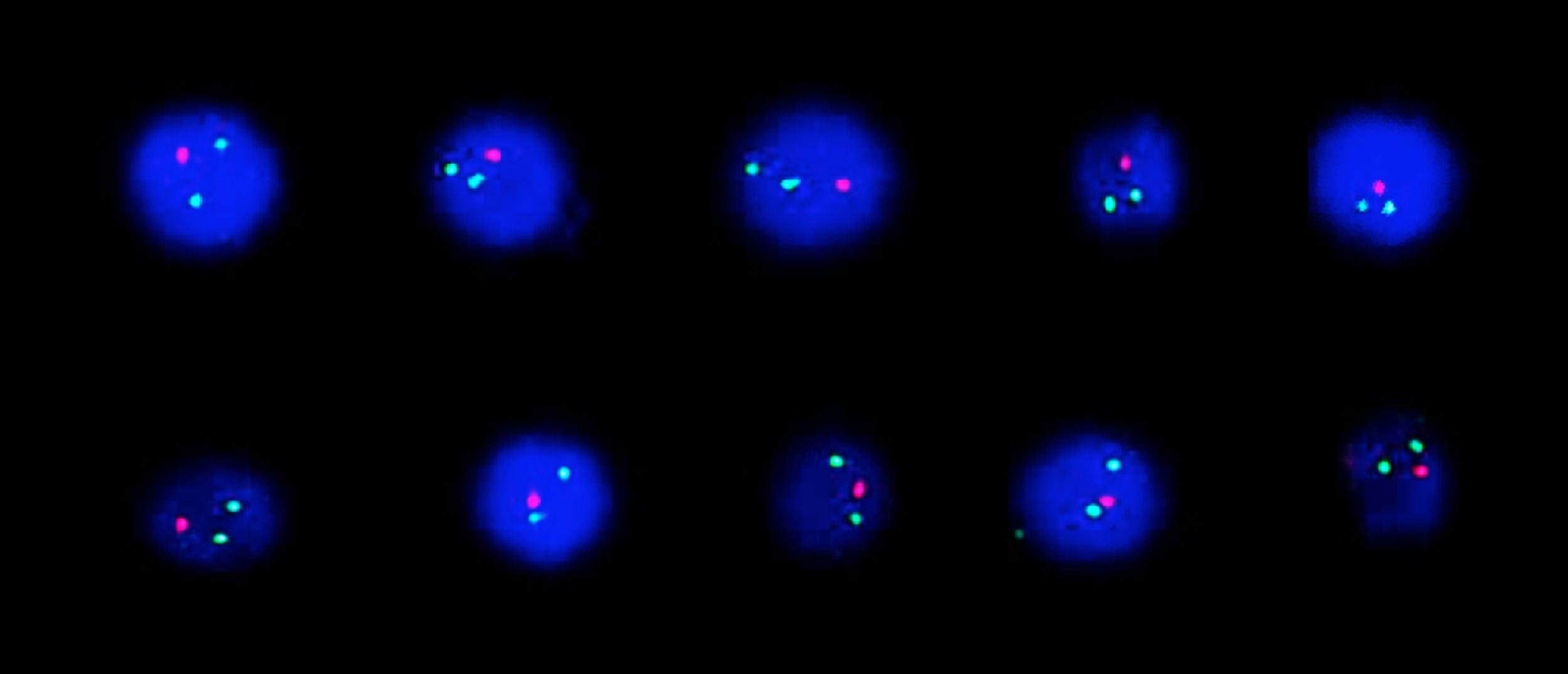
FISH analysis was performed using Zeiss Axio Imager.Z2 fluorescent microscope (Carl Zeiss MicroImaging GmbH, Munich, Germany) equipped with Metafer Slide Scanning System (MetaSystems GmbH, Altlussheim, Germany). A total of 200 interphase nuclei were scored. Analysis of BCR-ABL1 translocation dual fusion probe showed one red and two green signals in 90% of interphases indicating ABL1 deletion (Figure 2). No MYC rearrangement, IGH rearrangement KMT2A-AFF1, E2A-PBX1 or TEL-AML1 translocation were detected in examined interphases.
Follow upThe patient received induction chemotherapy. Post-induction day 15 bone marrow examination revealed 89% blasts. FISH was done using BCR-ABL1 dual fusion probe and revealed the same abnormality detected in baseline evaluation in 80% of examined interphases. The patient was lost to follow-up.
DiscussionABL1 deletion from chromosome 9 without t(9;22) has been previously reported in a very few cases of B-cell ALL.1,6-8 To the best of our knowledge, this is the first case of ETP-ALL with ABL1 deletion detected by FISH. It is not well known whether the ABL1 deletion in our case has a role in leukemogenesis or whether it represents an underlying genetic instability.
The main function of ABL1 is that it prevents the development of tumors by protecting the tumor suppressor gene p53 from degradation.9 When ABL1 gene is deleted, this results in degradation of tumor suppressor gene p53 and this contributes to leukemogenesis. ABL1 can also suppress the development of tumors by regulating the oncogenic transforming growth factor-β (TGF-β) signaling.10 ABL1 activation can block TGF-β tumor-promoting signals and thus, restoring a tumor-suppressing microenvironment. TGF-β up-regulates matrix metalloproteinases (MMPs) significantly, in especial MMP-9 and MMP-13, to enhance the invasion of tumor cells. ABL activation, however, can suppress the expression and secretion of MMPs through inhibiting TGF-β signaling. Therefore, this may be another mechanism to explain the occurrence of malignant tumors in patients with ABL1 deletion.8
ConclusionABL1 deletion was not previously reported in ETP-ALL. It is believed to be a very rare phenomenon. To the best of our knowledge, this is the first case of ETP-ALL, showing ABL gene deletion. Presence of ABL1 deletion may contribute to poor response to chemotherapy and therefore, the clinical significance of ABL1 gene deletion in ETP-ALL needs to be clarified.
Authors’ contributionAuthors Doaa F. Temerik, Walaa T. El-Mahdy and Ahmed Makboul analyzed the case. Author Ahmed Makboul wrote the manuscript. Author Doaa F. Temerik revised the manuscript.