Diversity in Classical Hematology Research
More infoImproving survival of Acute Lymphoblastic Leukemia (ALL) in adult patients has been a challenge. Despite intensive chemotherapy treatment, overall survival is poor. However, several studies demonstrate that young adult patients have better survival when treated with pediatric-based intensive regimens. Considering these results, We decided to treat newly diagnosed ALL patients according to age and risk factors. The goal of this study was to describe the results of this intensive chemotherapy treatment approach for ALL adult patients diagnosed at our institution.
MethodsFifty-eight ALL patients, diagnosed from 2004 to 2013, were included in the analysis. Patients were assigned to either the St. Jude Total Therapy XIIIB high-risk arm (St Jude) or the CALGB 8811 (CALGB). The Kaplan-Meier survival curve was used for the survival analyses and the Cox proportional hazard regression, for multivariable analysis.
ResultsThe overall survival was 22.9% at 10 years. The St. Jude improved survival, compared to the CALGB (p = 0.007), with 32.6% vs. 7.4% survival rate at 10 years. However, no survival benefit was found for patients younger than 20 years old (p = 0.32). The multivariable analysis demonstrated that undetectable minimal residual disease (MRD) and hematopoietic stem cell transplantation (HSCT) had beneficial impact on survival (p = 0.0007 and p = 0.004, respectively).
ConclusionALL is a disease of poor prognosis for adults. The joint effort to standardize treatment and seek solutions is the way to start improving this scenario.
Improving survival of Acute Lymphoblastic Leukemia (ALL) in adult patients has been a challenge for hematologists. Despite intensive chemotherapy, only 30 to 45% of patients maintain prolonged remission, compared to the excellent results after treatment in children.1,2,3,4 In the 21st century, intensive pediatric-inspired chemotherapy regimens demonstrated improved survival for older adolescents and young adults (AYA).3,5-7
The St. Jude Total Therapy XIIIB high-risk arm protocol is an example of an intensive chemotherapy regimen designed for pediatric patients in which post-remission therapy can be administered in an outpatient setting.6 This advantage is ideal for treating patients in our public health system, due to the scarcity of hospital beds for intensive chemotherapy management, as previously demonstrated by a Brazilian pediatric group.7
Reports on treatment results of adult ALL patients are sparse in Brazil. To our knowledge, the only available prospective cohort showed an overall survival (OS) of 41% at 5 years.8 On the other hand, a recent retrospective study of Brazilian adult ALL patients treated with a GMALL-based regimen showed a modest OS of 15.3% at 6 years.9 The main possible reasons for these results were delayed diagnosis, increased toxicity of intensive chemotherapy regimens and resources constraints in the Brazilian public health system.8,9
The survival of ALL patients at our institution until 2003 was 12 - 16% (unpublished data). Considering the best results of several pediatric-based intensive regimens to treat young adult ALL patients in the period between 2004 and 2013, our group decided to treat them with a pediatric-inspired chemotherapy or an adult-basis regimen, according to their age and risk factors. The objective of this report was to describe the results of this intensive chemotherapy treatment approach in a real-world setting for adult ALL patients diagnosed at the Hospital de Clínicas do Paraná (CHC-UFPR).
MethodsWe retrospectively reviewed 72 medical records of patients diagnosed with ALL and treated at the CHC-UFPR from 2004 to 2013. These patients had not been previously treated. In this period, patients older than 14 years were assigned to either the St. Jude Total Therapy XIIIB high-risk arm6 (St. Jude) or the CALGB 881110 (CALGB) regimen (Table 1), according to age and risk factors. The following criteria were used in assigning patients to the St. Jude arm:
- 1)
20 years old or younger;
- 2)
Low-risk disease if older than 20 years and younger than 35 years old.
St Jude high risk arm and CALGB regimens.
PDn = prednisolone; DNR = daunorubicin; VNC = vincristine; VP = etoposide; AraC = cytarabine; MTX = methotrexate; 6-MP = mercaptopurine; CY = cyclophosphamide; DOX = doxorubicin; 6-TG = thioguanine.
Criteria for low-risk disease were the following:
- 1)
< 30×109/L for B-lineage ALL and < 100×109/L for T-lineage ALL;
- 2)
Absence of central nervous system (CNS) infiltration;
- 3)
Absence of t(9;22), t(4;11) or hypodiploidy;
- 4)
Complete remission after induction;
- 5)
No previous chemotherapy for any cancer treatment, nor previous use of corticosteroids for prolonged time (case-by-case decision).
Patients older than 35 years old or patients with high-risk disease and age between 20 and 35 years old were assigned to the CALGB arm and evaluated for allogeneic hematopoietic stem cell transplantation (HSCT). Eight patients with mature B ALL (Burkitt's lymphoma) and two with mixed phenotype acute leukemia were excluded from the analysis. One patient who died before starting chemotherapy and three patients who were treated with other chemotherapy regimens were also excluded.
Patients with confirmed Philadelphia ALL (Ph+ ALL) were also treated with imatinib, as neither dasatinib nor ponatinib are available in the Brazilian public health system for first-line therapy. Non-remission patients after induction were assigned to salvage therapy. Patients unfit for chemotherapy were treated with supportive care, which consisted of corticosteroids, red blood cells and platelet transfusions and antibiotics.
The leukemia morphologic and multiparameter flow cytometry (MFC) classification was based on the WHO criteria.11-13 Conventional karyotyping and molecular analysis were performed to detect chromosomal abnormalities and the BCR-ABL fusion gene, respectively. The central nervous system (CNS) infiltration criteria were defined by the presence of at least 5 cells per cubic millimeter in the cerebrospinal fluid and confirmed MFC lymphoblasts.
Complete remission required normalization of peripheral counts (hemoglobin ≥ 7.0 g/dL, granulocyte count ≥ 1×109/L and platelet count ≥ 100×109/L) and no more than 5% blast cells in the bone marrow by day 28 of induction. Quantitation of minimal residual disease (MRD) was performed by 4 colors flow cytometry.14 A bone marrow blast count threshold of < 0.01% (10−4) was established for flow cytometry MRD assessment. Although the MRD was routinely tested, it was not utilized for the purpose of treatment consolidation with HSCT. There were no robust data in that period that detectable MRD in adult patients was a solid marker of high-risk disease and best managed with HSCT.
All the information was extracted from the Hospital Information System.
Descriptive statistical analyses were performed with the Excel 2016 and EZR v1.35 programs. Survival curves were plotted with the Kaplan-Meier method and were compared with the log-rank test. Multivariable analysis with the Cox proportional hazard regression model was used for survival.
This study was approved by the CHC-UFPR Ethics Committee under the Certificate of Presentation for Ethical Consideration (CAAE) number 77963217.9.0000.0096.
ResultsPatient population and demographic dataA total of 58 patients with acute lymphoblastic leukemia were evaluated retrospectively over a 10-year period. These patients were treated with either the St. Jude (n = 35) or the CALGB (n = 21) regimens, depending on their age and disease risk at diagnosis. Two patients received only supportive treatment.
The cohort was mostly comprised of men: 33 patients (56.8%). In addition, the patients were predominantly young: median age of 26 years (range = 15 – 81), eighteen patients were 20 years old or younger. B-cell and T-cell lineages were found in 49 and 9 patients, respectively. The most frequent immunophenotype found was the Common B ALL (n = 47).
There were many patients with high-risk cytogenetics: two had t(4;11), six had t(9;22) and two had hypodiploidy. Other cytogenetics of interest consisted of t(1;19) and hyperdiploidy (n = 3 and n = 5, respectively). The presence of Ph+ ALL was confirmed by molecular analysis in 12 patients, six of whom presented only the transcript, with normal cytogenetics or without metaphases. Finally, the p190 transcript was present in 9 patients and the p210, in three. Nonetheless, 16 of the 49 (32%) B-lineage ALL patients were not tested for the BCR-ABL mutation.
The only significant difference between treatment groups was age (p < 0.0001). Population characteristics are shown in table 2.
Baseline patients’ demographic and clinical characteristics.
WBC: white blood count; CNS: central nervous system; NA: not available. Data described in number and percentage.
Thirty-five patients achieved complete response after induction (62.0%): 23 in the St. Jude arm (65.7%) and 12 in the CALGB arm (57.1%). Of these, 15 patients achieved undetectable MRD (25.85%): eleven (31.4%) in the St. Jude arm and four in the CALGB arm (19.0%). Data on the MRD were missing from the medical records of 4 patients. Moreover, five patients in the St. Jude arm and two patients in the CALGB arm failed induction therapy and were reassigned to salvage treatment.
Eight patients died during the induction therapy, six in the CALGB arm and two in the St. Jude arm. Of these, five died of septic shock, four in the CALGB arm and one in the St. Jude arm. One patient died of pulmonary hemorrhage and other two, of unknown causes.
Both patients who received supportive treatment died two months after diagnosis, one of septic shock and the other of an unknown cause.
Nine patients underwent HSCT (15.5%). Six had high-risk cytogenetics: four Ph+ and two t(4;11). The indication for HSCT for the other 3 patients were induction failure (one patient) and age (two patients).
SurvivalIn total, 14 patients are alive. Four of them underwent HSCT: two Ph+ ALL, one T-lineage ALL and one t(4;11). Interestingly, the latter received the CALGB regimen and was 34 years old at diagnosis, while the other two Ph+ ALL living patients received the St. Jude regimen.
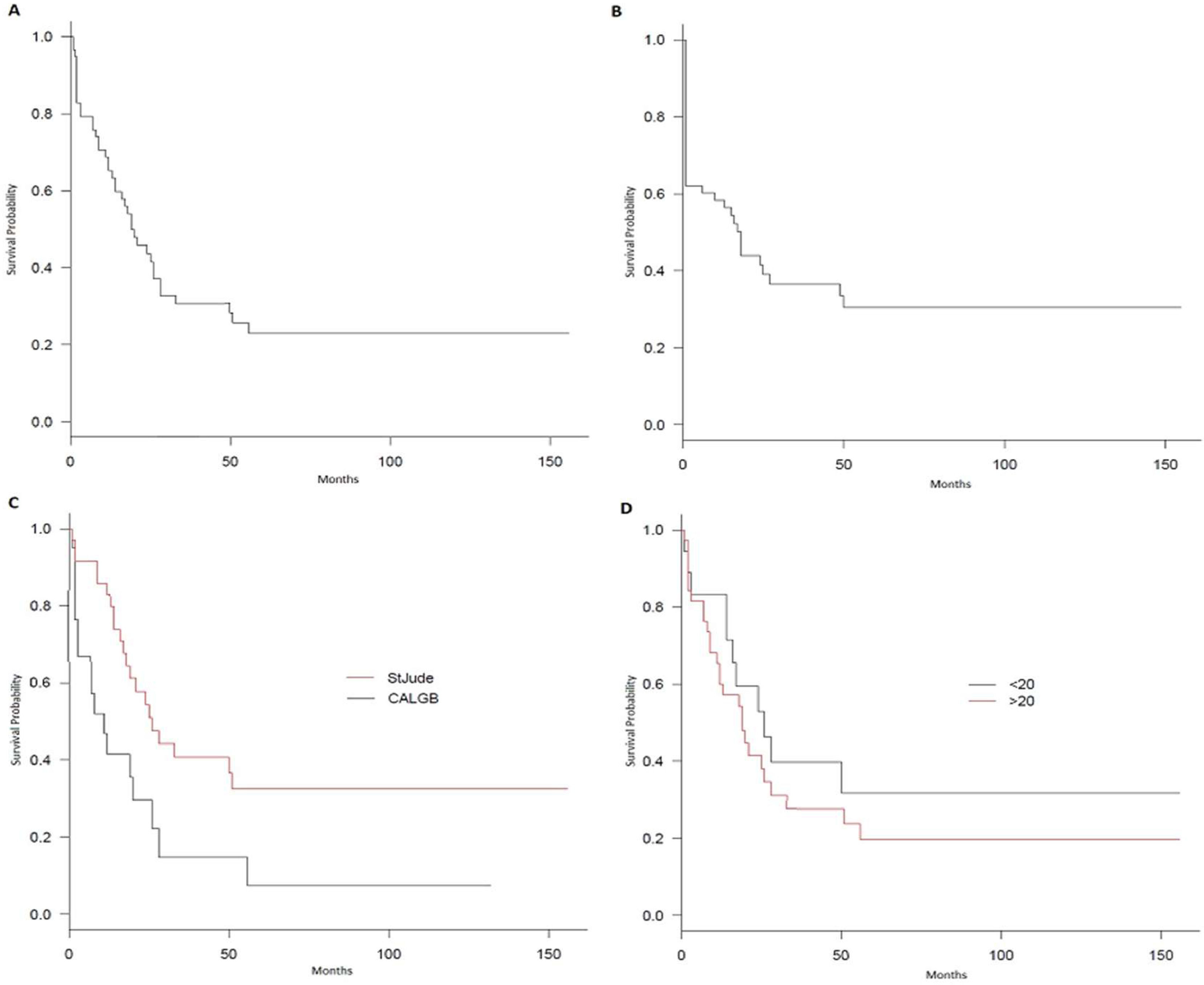
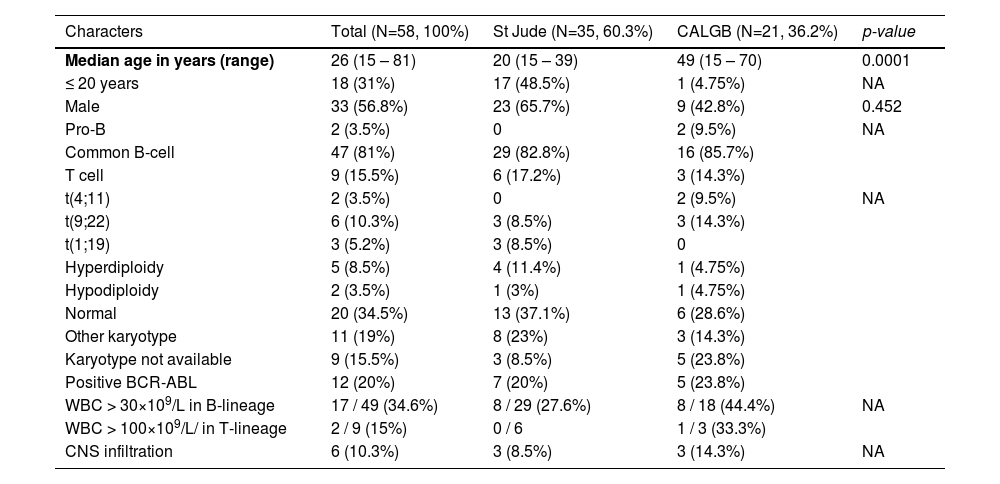
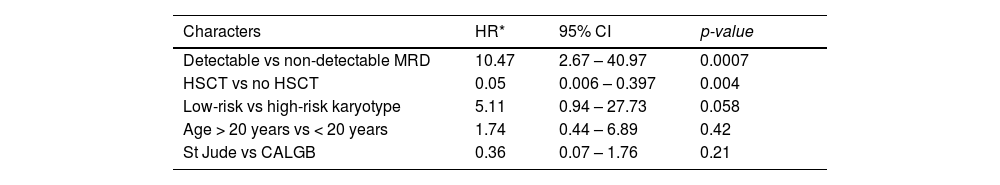
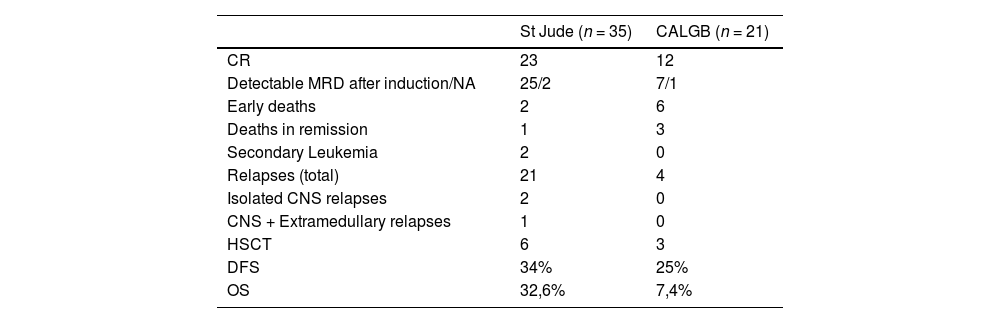
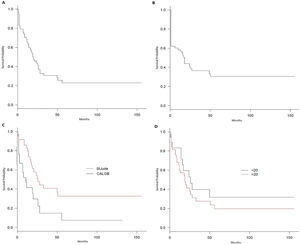
The OS was 22.9% at 10 years, with a median of 20 months (Figure 1A), and the disease-free survival (DFS) was 30.5% in 10 years, with a median of 18 months (Figure 1B). The St. Jude had better survival, compared to the CALGB regimen (95% CI 17 – 51 vs. 3 – 26; p = 0.007), with a 32.6% vs. 7.4% survival rate at 10 years (Figure 1C). However, no survival benefit was found for patients younger than 20 years old versus older than 20 years old (95% CI: 14 – NR vs. 11 – 28; p = 0.32), as shown in Figure 1D.
Multivariable analysis showed that only undetectable MRD (HR: 10.47; 95% CI: 2.67 – 40.97; p = 0.0007) and HSCT (HR: 0.05; 95% CI: 0.006 – 0.397; p = 0.004) had beneficial impact on survival (Table 3).
Multivariable analysis of Overall Survival.
| Characters | HR* | 95% CI | p-value | |
|---|---|---|---|---|
| Detectable vs non-detectable MRD | 10.47 | 2.67 – 40.97 | 0.0007 | |
| HSCT vs no HSCT | 0.05 | 0.006 – 0.397 | 0.004 | |
| Low-risk vs high-risk karyotype | 5.11 | 0.94 – 27.73 | 0.058 | |
| Age > 20 years vs < 20 years | 1.74 | 0.44 – 6.89 | 0.42 | |
| St Jude vs CALGB | 0.36 | 0.07 – 1.76 | 0.21 |
Of the 58 patients, 44 died (75.8%). The main cause of death was disease relapse (25 patients, 21 in the St. Jude arm and 4 in the CALGB arm – 43.1%), herein included all five patients who failed the St. Jude induction therapy and one patient who failed the CALGB induction. There were two isolated CNS relapses (both T lineage ALL with high leukocyte counts and CNS infiltration, respectively, at diagnosis) and one CNS and extramedullary relapse (B lineage ALL, without high-risk features at diagnosis), all in the St. Jude arm.
Most relapsed patients (11 in the St. Jude arm, including those with CNS relapses and 2 in the CALGB arm) did not achieve undetectable MRD after the first induction therapy and only two underwent HSCT (one in each treatment arm). There is no entry in the medical records describing why the others did not receive bone marrow transplantation. Septic shock was the second most frequent cause of death (8 patients – 13.8%), three in the St. Jude arm, four in the CALGB arm and one in the supportive treatment group. Other causes of death were secondary acute myeloid leukemia (two deaths, both in the St. Jude arm), pulmonary hemorrhage (one death) and chronic graft versus host disease complications in one transplanted patient. Seven had unknown causes of death (12.0%).
Table 4 summarizes the described outcomes.
Outcomes.
CR: complete response; MRD: minimal residual disease; NA: not available; CNS: Central nervous system; HSCT: allogeneic hematopoietic stem cell transplantation; OS: overall survival; DFS: Disease-free survival. Data described in number and percentage.
Acute lymphoblastic leukemia in adults and particularly in the AYA population (15 to 39 years) remains a difficult clinical problem, especially regarding therapy selection.3,4 Data related to results of intensive pediatric regimens, compared to standard adult chemotherapy, are still growing, yet differences in disease features, response to treatment and social factors may be responsible for the worse prognosis in this group.3 On the other hand, although cytogenetic and clinical characteristics matter, response measured by the MRD and access to transplant are crucial to survival in this population.1,2
In this article, Brazilian ALL patients were evaluated retrospectively over a 10-year period and received either an intensive pediatric approach based on the St. Jude protocol or an adult-basis protocol proposed for the CALGB group, depending on their age and disease risk at diagnosis. The St. Jude treatment arm was comprised of younger patients and lower-risk disease, while the CALGB arm was the opposite. Approximately one-third of the cohort were under 20 years of age, most of them (n = 17) in the St. Jude arm.
Although there was no difference in the induction response rate between the two treatments, the St. Jude intensive post-remission chemotherapy improved survival, compared to the CALGB regimen, with a 32.6% vs. 7.4% survival rate at 10 years. However, no survival benefit was found for patients younger than 20 years old. This lack of benefit might have occurred as a combined result of mortality due to septic shock, low number of bone marrow transplants despite detectable MRD after induction and, consequently, a higher number of disease relapses. The St. Jude induction phase was highly toxic, with prolonged aplasia and some early deaths. Likewise, the unexpected DFS rate derived from the high number of non-relapse deaths.
Of note, there were two isolated CNS relapses and one CNS plus extramedullary relapse in the St. Jude arm. Although the St. Jude Total Therapy XIIIB intensive intrathecal chemotherapy markedly reduced the CNS relapse rate,5,15 very late cranial irradiation (at week 56) may be responsible for the relapses, including this cohort, especially if high-risk features at diagnosis are present.15
The multivariable analysis demonstrated that undetectable MRD and access to bone marrow transplantation favored survival, which is in accordance with the international literature,1 although this effect was not seen for the low-risk karyotype and the St. Jude regimen. However, the HSCT advantage must be interpreted with caution, as this result may be due to selection bias. The absence of karyotyping and MRD testing for some patients (including living St. Jude-treated patients) precluded better assessment of risk factors and their influence on survival. Additionally, probably related to high etoposide dose exposure, there were two secondary cases of acute myeloid leukemia in the St. Jude group, which is in accordance with long-term St. Jude Total Therapy XIIIB results.15
It must be emphasized that testing for the BCR-ABL fusion gene (p190 and p210) and high-risk karyotypes is of utmost importance, as these patients are prone to relapses and worse outcomes. Furthermore, the HSCT might overcome these adverse risk factors, as in this cohort the HSCT favored a better outcome. MRD testing is also extremely important. Currently, the MRD is a well-stablished independent risk-factor of worse prognosis, also predicting relapse and decreased survival.16,17 We acknowledge that the HSCT should have been offered to those with detectable MRD, however, robust information on the prognosis in this MRD setting was lacking in the period analyzed in this article. Other authors also published their difficulties in interpreting survival data in the absence of reports on the MRD in an analyzed period when its role was not very well defined either.18 Better assessment of survival between detectable vs. undetectable MRD may have been precluded.
The result of this study is in agreement with several others that demonstrated the same relation between intensive therapy and better survival in adult patients.3,15,19,20 In the same manner, the overall response of 22.9% was better than the previous period mentioned (12 - 16% in the early 2000´s). Conversely, the percentage of survival in those other studies is higher, when compared to the Brazilian experience.8,9 This discrepancy could be explained by the lack of resources in public hospitals for better supportive measures, such as availability of broad-spectrum antibiotics and intensive care unit beds for septic shock treatment. Complex social, economic and psychological factors may have affected therapy adherence and influenced survival as well and those are also concerns of other low-income countries, as shown by the Indian Hematology Cancer Consortium group's correspondence.21 Furthermore, we cannot know if the Philadelphia-like ALL has influenced the outcomes. This group of mutations is more frequent in Latin ethnicity and confer a poor prognosis.22,23 However, molecular tests are not widely available in Brazil for testing these mutations and consequently, not commonly reported. Finally, a retrospective analysis is the major limitation of this report, which may have led to selection bias and favored the intensive pediatric-based chemotherapy regimen.
ConclusionAcute lymphoblastic leukemia is still a disease of poor prognosis for the adult population. Especially in our country, pediatric-inspired intensive chemotherapy and wide population access to bone marrow transplantation are still challenges for the Brazilian public health system. The joint effort of several centers to standardize treatment and seek solutions is the way to start improving this scenario.