During pregnancy, the iron requirement increases to meet the optimal growth of the fetus and prevent iron deficiency anemia-related complications in the mother. However, in sickle cell disease (SCD) primarily due to repeated blood transfusions and hemolysis-induced recycling of iron, its supplementation during pregnancy remains questionable and may be harmful.
MethodsTwenty-five pregnant women with homozygous SCD and 25 pregnant women with normal hemoglobin variants were included as cases and control, respectively. Pregnancy and sickle cell anemia (SCA) were diagnosed using standard protocols. The serum iron, serum ferritin, total iron-binding capacity (TIBC), percentage transferrin saturation and C-reactive protein were estimated, as per the manufacturer's protocol. The complete blood count was performed. The unpaired ‘t-test’ was performed using the SPSS v23.0 and the principal component analysis (PCA) was performed using the online software MetaboAnalyst for statistical analysis.
Main ResultsThe studied cases had significantly lower mean hemoglobin and higher mean corpuscular volume (MCV), compared to controls. The mean serum-iron, serum-ferritin and percentage transferrin-saturation in the cases were significantly higher than that of the controls, while the TIBC was lower in the cases (p < 0.0001). The mean level of serum iron, ferritin, percentage transferrin saturation and TIBC were 309.44 ± 122.40mcg/dl, 860.36 ± 624.64ng/ml, 42.6 ± 17.30% and 241.32 ± 96.30 mcg/dl, respectively, in the cases and 95.36 ± 41.90mcg/dl, 122.28 ± 49.70ng/ml, 15.83 ± 3.10% and 492.6 ± 149.40mcg/dl in the controls, respectively. Higher MCV, mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) with lower hemoglobin (Hb) were noted in the cases. The PCA revealed that the cases were more heterogeneous in terms of the variability of the iron status and hematological indices than the controls.
ConclusionThe current study shows iron sufficiency in most cases of pregnancy with SCA and suggests that evaluation of iron status must be made before initiating iron prophylaxis in pregnant women with SCA, especially in regions having a high prevalence of sickle cell hemoglobinopathy.
Sickle Cell Disease (SCD) is an autosomal recessive disease characterized by transversion mutation in the second nucleotide of the sixth codon (GAG to GTG) of the β-globin gene. India is the second most affected country, with an estimated frequency of SCD baby birth of 42,016 in 2010.1 The highest predicted sickle gene frequency, prevalent both in tribal and nontribal populations across central India, was found in western Odisha. Clinically, the SCD phenotype varies from asymptomatic to severe forms with multi-organ damage and even premature death.2
Iron is an essential nutrient for pregnant women and the developing baby, both in India and in affluent countries; its requirement is increased during pregnancy, more so in the second half.3,4 The iron absorbed in the small intestine is transported by transferrin to the bone marrow for the production of hemoglobin and excess iron is stored as ferritin intracellularly. A small part of ferritin is secreted into the blood. Iron deficiency anemia (IDA) is one of the most common nutritional problems and about 52% of pregnant women are affected with IDA in developing countries. 5,6 In India, due to the difference in the nutritional status, the prevalence of iron deficiency varies and has been reported to be 20% to 48.4% in Tamil Nadu State and 73.4% in Hariyana State among pregnant women.7,8 The daily iron and folic acid supplementation recommended for pregnant women by the World Health Organization (WHO) is 60mg elemental iron and 400µg folic acid.9
The commonly used parameters for iron status evaluation are serum iron, serum ferritin, total iron binding capacity (TIBC) and percentage transferrin saturation. The serum iron reflects the amount of iron bound to transferrin and available for use by marrow and other tissues of the body, but is not indicative of iron stores and is used mainly for calculation of percentage transferrin saturation. Serum ferritin levels correlate well with body iron stores and reflect iron deficiency at levels ≤ 30ng/ml.10,11 Serum ferritin indicates iron overload when > 300ng/ml in males and > 150 - 200 ng/ml in females, in the absence of inflammation and liver disease.12 The TIBC is an indirect measure of transferrin; it indicates iron deficiency when increased and iron overload when decreased.13 The percentage transferrin saturation > 50% suggests increased iron delivery to non-erythroid tissues and iron overload.
In SCD patients, when the red blood cells are hemolyzed, it is assumed that iron components of the heme molecule that constitutes the hemoglobin are stored and reused because there is no active hemorrhage taking place. Hence, these patients are not likely to be iron deficient even though they have chronic anemia.14 Furthermore, iron excess may lead to increased morbidity and mortality in SCD.15 Extrapolation of these facts would lead to the assumption that pregnant women with SCD should not suffer from iron deficiency. However, it has been reported that about one-third of the hemolysis is intravascular and loss of iron in urine can occur through hemoglobinuria.16-18 Other factors, such as poor nutrition and parasitic infestations, may interplay in the development of the IDA in SCD patients.19,20 In a study from India, the IDA was reported in 67% of sickle cell anemia (SCA, HbSS) cases,21 whereas studies from Nigeria in children aged under five with SCA mentioned higher iron stores.22 Higher iron levels were noted in pregnant SCD women in a study from Benin City, Nigeria, thus yielding conflicting results in different parts of the world.23 This study was therefore undertaken to evaluate the iron profile and resolve this ambiguity in pregnant Indian women with SCA, as well as to clarify the role of iron prophylaxis in them.
Materials and MethodsThis was a hospital-based case-control study conducted at Veer Surendra Sai Institute of Medical Science And Research (VIMSAR), Burla, Odisha State, India from November 2016 to November 2018. The study subjects were among those admitted to the department of Internal Medicine and the Department of Obstetrics and Gynecology or attended to in their regular antenatal checkup. The cases and controls were selected as per the inclusion and exclusion criteria mentioned below.
Inclusion and Exclusion criteriaPregnant women with SCA and pregnant women with normal hemoglobin (HbAA) variants were included in the study as ‘cases’ and ‘controls’, respectively. Pregnant women affected with other hemolytic diseases, such as thalassemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency, those with a history of blood transfusion (BT) within the last three months, those with a history of repeated transfusion ≥ 5 units of blood, those with raised C-reactive protein (CRP) levels (> 5 mg/l), subjects with liver disease, patients in painful crises and patients taking hydroxyurea, were excluded from the study.
Informed consent was obtained from all the study subjects and their data were recorded in predesigned cases record proforma. Demographic and clinical profiles including the history of transfusion were recorded; diagnostic and laboratory evaluation including the complete blood count (CBC) and iron profile study of the cases and controls were performed as mentioned below.
Diagnosis of pregnancyPregnancy was suspected by the presence of the β-human chorionic gonadotropin (β-hCG) in the morning urine sample after a missed period. Detection of the β-hCG was obtained by the strip method. The women's morning urine sample was collected and kept in a container. The test strip was vertically submerged in urine to the marked end of the arrows for five seconds then kept on a dry flat surface. A positive result gives two lines, the control line and result line indicate that the urine contains β-hCG. Subsequently, confirmation of pregnancy was provided by the presence of the gestational sac or intrauterine fetus determined by a certified ultrasonologist.
Diagnosis of SCDThe SCD was diagnosed using a three-step method of sickle slide tests, the Hb electrophoresis by alkaline agarose gel and cation exchange high-performance liquid chromatography (CE-HPLC). The CE-HPLC was used to detect and quantify various Hb fractions based on individual retention time (RT) using the Variant-II β-thalassemia Short Program Fully Automated Hemoglobin Testing System (Bio-Rad Inc. Hercules, CA, USA). Those with sickle red cells found on the slide test, sickle hemoglobin (HbS) or (HbSF) band in Hb electrophoresis and an HbS over 50% with HbF in the CE-HPLC, were diagnosed as having SCD. They were further classified as homozygous (HbSS) or compound heterozygous states depending on the presence of various HbS and other Hb variants. Homozygous SCD (HbSS) was diagnosed when the CE-HPLC showed HbS (60 - 85%), HbF (10 - 15% or more) and HbA2 (1.5 - 3.5%), in addition to sickle cells in peripheral blood and HbS or (HbSF) band in hemoglobin electrophoresis.
Hematological and biochemical parameter analysis including iron profileHematological indices were analyzed by the Sysmex KX21 Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan). The biochemical parameters analyzed were serum iron, ferritin, total iron binding capacity (TIBC), percentage transferrin saturation and CRP. The serum iron and TIBC estimations were obtained using the VITROS Fe Slide and VITROS TIBC Slide by Chemiluminescence methods (Ortho-Clinical Diagnosis, Inc, Rochester, New York, USA). The serum ferritin concentration was estimated by quantitative enzyme-linked fluorescent immunoassay (ELFA) using the VITROS 5600 (Ortho-Clinical Diagnosis, Inc, Rochester, New York, USA). The percentage of transferrin saturation was calculated using the formula serum iron level multiplied by 100 and divided by the total iron binding capacity by the pre-installed software of the autoanalyzer. The CRP was estimated by the immunoturbidometric method in the AU480 (DiaSys diagnosis systems, Holzheim, Germany), as per the manufacturer instructions.
All procedures performed in this study involving human participants were performed following the ethical standards of the institutional ethical committee of VIMSAR, Burla, Odisha (VIREC No.2016/I-F-CT-01/033), as well as the 1964 Helsinki declaration recommendations and their later amendments.
Statistical AnalysisFor comparison between controls with the normal hemoglobin variant (HbAA) and the cases with SCA (HbSS), an unpaired t-test was performed on the GraphPad InStat version 3.0 and the SPSS version 23.0 (Statistical Package for Social Sciences, Inc, Chicago, III). The level of significance was considered when the p-value was < 0.05. The principal component analysis (PCA) was performed using the MetaboAnalyst online software (www.metaboanalyst.ca).
ResultsAll 25 cases in the present study were found to have homozygous SCD (SCA, HbSS). The controls were pregnant women with a normal hemoglobin variant. In the current study, all the subjects (cases and controls) were in the age range of 20 to 30 years, the median age of the cases being higher than that of the controls (27 vs. 22 yrs). Ninety-two percent of the cases and 64% of the controls came to our hospital in the third trimester of pregnancy (Table 1). As per the national policy in India, all pregnant mothers are provided with iron (100mg, elemental iron) and folic acid (0.5mg) daily supplementation from the second trimester onwards. Hence, all ‘cases’ and ‘controls’ except one control were on daily iron-folic acid supplementation at the time of inclusion in our study.
Demographic profile, gestational profile, iron profile indices and C-reactive protein level of pregnant women with SCA and normal hemoglobin variant.
| Cases | Controls | p-value | |
|---|---|---|---|
| Age | |||
| Median | 27 | 22 | 0.0003* |
| 20 - 25 years | 6 (24%) | 20 (80%) | |
| 25 - 30 years | 19 (76%) | 5 (20%) | |
| Gestational age | |||
| Median | 32 | 32 | 0.4222 |
| 1st trimester | 0 (0%) | 1 (4%) | |
| 2nd trimester | 2 (8%) | 8 (32) | |
| 3rd trimester | 23 (92%) | 16 (64%) | |
| Serum iron (normal level 60 - 80mcg/dl) | |||
| Mean ± SD | 309.44 ± 122.40 | 95.36 ± 41.90 | < 0.0001* |
| Less than normal | 0 (0%) | 6 (24%) | |
| Within normal | 5 (20%) | 19 (76%) | |
| More than normal | 20 (80%) | 0 (0%) | |
| Serum ferritin (normal level 9.3 - 159ng/ml) | |||
| Mean ± SD | 860.36 ± 624.64 | 122.28 ± 49.70 | < 0.0001* |
| Less than normal | 0 (0%) | 0 (0%) | |
| Within normal | 3 (12%) | 25 (100%) | |
| More than normal | 22 (88%) | 0 (0%) | |
| TIBC (normal level 250 - 450mcg/dl) | |||
| Mean ± SD | 241.32 ± 96.30 | 492.6 ± 149.40 | < 0.0001* |
| Less than normal | 13 (52%) | 0 (0%) | |
| Within normal | 11 (44%) | 13 (52%) | |
| More than normal | 1 (4%) | 12 (48%) | |
| %Transferrin saturation (normal level 11 - 50%) | |||
| Mean ± SD | 42.60 ± 17.30 | 15.83±3.10 | < 0.0001* |
| Less than normal | 0 (0%) | 0 (0%) | |
| Within normal | 14 (56%) | 25 (100%) | |
| More than normal | 11 (44%) | 0 (0%) | |
| C-Reactive Proteins (normal level 2-5 mg/l) | |||
| Mean±SD | 2.8 ± 1.12 | 3.00 ± 0.97 | > 0.05 |
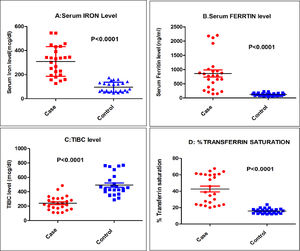
The mean serum iron and serum ferritin were significantly higher (p < 0.0001) in the cases, as compared to the controls (309.44 ± 122.40mcg/dl in cases vs. 95.36 ± 41.90mcg/dl in controls for serum iron and 860.36 ± 624.64ng/ml in cases vs. 122.28 ± 49.70ng/ml in controls for serum ferritin). When both the parameters were considered, more than 80% of the cases had their levels above the normal reference range, while none of the controls had their values above the normal reference range (Table 1; Figure 1A and 1B). Six of the cases (24%) had serum ferritin > 1000 ng/ml and of these, three cases (12%) had serum ferritin > 2000 ng/ml (Figure 1B).
Distribution of various parameters in the cases and controls. A: Serum Iron level in mcg/dl (normal level 60-80 mcg/dl); B: Serum ferritin level in ng/ml (normal level 9.3-159 ng/ml); C: TIBC in mcg/dl (normal level 250-450 mcg/dl); D: Percentage of transferrin saturation (normal level 11-50%).
Serum CRP levels of all the study subjects were within the normal reference range, with no significant difference among the cases and controls (2.8 ± 1.12mg/dl in cases and 3.0 ± 0.97mg/dl in controls) (Table 1). Since none of the subjects had an elvevated CRP, the effect of inflammation on the estimation of serum ferritin was nullified.24 The mean number of transfusions in the cases received in past was 1.8 ± 0.86 (range 0 to 3), while in the controls it was 0.6 ± 0.58 (range 0 to 1).
There was a statistically significant (p < 0.0001) lower mean TIBC level in the cases (241.32 ± 96.30mcg/dl), as compared to the controls (492.6 ± 149.40mcg/dl). Only one (4%) of the cases had a TIBC above the normal references range, while nearly half of the controls had a TIBC above the normal range (4% vs. 48%). Moreover, the majority of the cases (52%) had a TIBC below the normal range, as depicted in the scatter diagram of Figure 1C and in Table 1.
As shown in Table 1, significantly (p < 0.0001) higher mean values were observed for percentage transferrin saturation in the cases, compared to the controls (42.6 ± 17.30% in cases and 15.83 ± 3.10% in controls). Among the cases, 44% had percentage transferrin saturation above the normal reference range, compared to none in the controls. None of the cases had a percentage of transferrin saturation below normal (Table 1and Figure 1D).
Hematological indicesThe mean Hb level of the cases (7.8 ± 0.62gm/dl) was significantly (p < 0.001) lower than that of the control group (12.46 ± 1.00gm/dl). The mean MCV of the cases (93.1 ± 4.99fL) was significantly (p < 0.001) higher, as compared to the controls (81.0 ± 21.50 fL). The observed mean corpuscular hemoglobin (MCH) between the groups was nearly equal (30.65 ± 3.50pg for cases and 30.15 ± 3.48pg for controls). The mean corpuscular hemoglobin concentration (MCHC) was marginally higher in the cases (33.66 ± 1.50 gm/dl) than that of the controls (32.91 ± 1.48 gm/dl) although the difference was not significant (p > 0.05). Pregnant women with SCA had a significantly higher (p < 0.0011) total leucocyte count (TLC), as compared to the controls (10,734 ± 4,487 per cubic mm vs. 4,854 ± 1,687 per cubic mm). The mean total platelet count (TPC) was lower in the cases, as compared to the controls (2.1 ± 0.76lacs per cubic mm vs. 2.58 ± 1.01lacs per cubic mm), but the observed differences between the groups were not significant (p > 0.05), (Table 2).
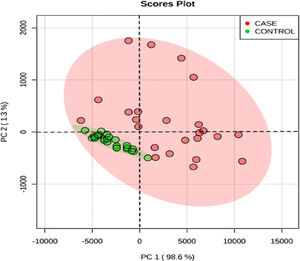
The score plot graph of the principal component analysis (PCA) of all variables of the entire data shows a different pattern of distribution between the cases and the controls (Figure 2). The distribution is more uniform in the controls and heterogeneous in the cases.
Hematological indices of pregnant women with SCA and normal hemoglobin variant.
| Cases | Controls | p-value | |
|---|---|---|---|
| MEAN ± SD | MEAN ± SD | ||
| Hb (gm/dl) | 7.8 ± 0.62 | 12.46 ± 1.00 | < 0.001* |
| MCV (femtolitres) | 93.1 ± 4.99 | 81.02 ± 1.50 | < 0.001* |
| MCH (picograms) | 30.65 ± 3.50 | 30.15 ± 3.48 | > 0.05 |
| MCHC (gm/dl) | 33.66 ± 1.50 | 32.91 ± 1.48 | > 0.05 |
| TLC/cubic mm | 10734 ± 4487 | 4854 ± 1687 | < 0.0011* |
| TPC (lakhs/cubic mm) | 2.1 ± 0.76 | 2.58 ± 1.01 | > 0.05 |
MCV: Mean Cell Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; TLC: Total Leukocyte Count; TPC: Total Platelet Count.
In the current study, all the cases were in the age range of 20 to 30 years. Ogbimi BN et al. in Nigeria reported 65.2% of the pregnant women with SCD (HbSS and HbSC) having ages between 20 to 30 years, whereas the rest of their cases were older than 30 years.23 Most of the study subjects came to our hospital in the third trimester of pregnancy. Most of the subjects were having the antenatal checkup in peripheral health institutions near their home in the first and second trimesters, but came to our hospital (with a tertiary care facility) either on their own or on referral in the third trimester or nearer to term for evaluation of risk and/or safe confinement.
On observations of higher serum iron, serum ferritin, raised percentage transferrin saturation and low TIBC in pregnant women affected with SCA in the present study it is appropriate to state that they are in the iron repleted state. 14,25,26 The mean serum ferritin in this study is found to be higher (860 ng/ml) in pregnancy with SCA than reported in the study done by Ogbimi BN et al. (120ng/ml) at similar gestational age.23 Abudu OO et al. also found higher serum ferritin in their cases of pregnant HbSS or HbSC women, compared to pregnant controls with HbAA (680µg/L vs. 79.94µg/L) at 28 weeks of gestation.20 In the present study, six out of 25 cases had serum ferritin above 1000ng/ml, three of whom had a level > 2000ng/ml, indicative of an iron overload state. Hence, iron supplementation was stopped in these six pregnant SCA women. Most of these cases had had transfusions limited to three in the past. The SCA patients transfused with < 5 units of blood had a similar iron status compared to those never transfused.27,28 Coexisting inflammation leading to an elevated ferritin level was excluded by the normal CRP level.24 In an earlier study by Oluboyde O.A, the difference in levels of serum ferritin between pregnant and non-pregnant HbSS and HbSC women was not significant. 29 A few other studies also found no source of iron in the bone marrow of pregnant mothers with SCD.30,31 Such differences may be due to variations in methodology and samples studied, or the difference in patterns of iron intake in the study subjects. As the study subjects were comparatively younger, the variation in serum ferritin with increasing age was minimized.32 The iron requirement increases during pregnancy due to an increased demand by both the growing fetus and the mother and the role of iron prophylaxis can not be overemphasized.3,4 However, it was the view of some studies that iron overload in SCD patients had adverse consequences in many vital organs.15,33-35 Nevertheless, SCD patients with low levels of adult hemoglobin have less occurrence of hypoxia-induced hemolysis, thus ameliorating the hemolytic crisis. 32,36 The percentage transferrin saturation above 50% was observed in 44% of the cases in the present study. This is in contrast with the observations made by Mohanty et al. involving non-pregnant SCD patients in which 10.8% of SCD patients had percentage transferrin saturation above 50%.26 Most of the cases had a TIBC below normal or within the range, with only 4% being above the normal range, suggestive of the absence of an iron deficiency state in most of the pregnant women with SCD. The current study suggests that iron supplementation in pregnant sickle cell mothers is justified only after evaluation of the adequacy of iron stores, as values are more widely distributed than those of the controls. Guidelines also suggest in favor of iron status evaluation in pregnancy before initiating iron prophylaxis.37
Pregnant women with SCA had lower levels of Hb and higher MCV, compared to pregnant women with a normal hemoglobin variant. The same results were found in earlier studies involving pregnant sickle cell mothers.14,23 However, some studies were conducted involving nonpregnant SCD women with a similar findings of lower Hb and higher MCV, MCH, and MCHC in SCD, as compared to women without SCD.38 The low Hb observed in pregnant SCD patients may be due to chronic hemolysis with the subsequent reduction in the number of red blood cells (RBCs), causing normal MCV and MCH. The markers of hemolysis, however, were not evaluated in the present study. Although the total leukocyte count is higher in cases than in controls,. this is a usual finding in the steady state of SCD. The mean leukocyte count found in the steady state of SCA ranges from 12 to 15×109/L with a range from 6 to 20×109/L due to shifting of granulocytes from the marginated to the circulating pool.39 The total platelet count in the cases and controls in this study was within the normal limit.
Homogeneous distribution in the PCA score plot for control and heterogeneous distribution in sickle cell pregnant women reflects a greater phenotypic variation in SCA patients, with varying clinical and biochemical characteristics. This also indicates that the iron parameters are different in different phenotypes of SCA with pregnancy and hence there is a need for individualization of iron supplementation.
ConclusionIron prophylaxis is essential in most women without SCA during the pregnancy; however, in the case of pregnant SCA women, this may be need-based, as many have normal or more than normal iron stores and iron deficiency is rare. Therefore, the best approach is to estimate serum iron, ferritin, TIBC and percentage transferrin saturation to know the iron status of the SCA pregnant women in the first trimester to verify if the patients need iron or not. Recently several other parameters being evaluated are the soluble transferrin receptor, reticulocyte hemoglobin and serum hepcidin level, which may complement or replace these conventional iron profile parameters in the future.
The authors are thankful to the Director, Veer Surendra Sai Institute of Medical Science and Research (VIMSAR), Burla, Sambalpur, Odisha (VIMSAR) and the Dean and Principal, VIMSAR for their kind support and necessary official approvals.