Systemic Mastocytosis is a rare myeloproliferative neoplasm characterized by an increased number of neoplastic mast cells in the extracutaneous organs, with the bone marrow being the most frequent site of involvement. The 2016 World Health Organization (WHO) Classification of Tumors of Haematopoietic and Lymphoid Tissues recognizes that Systemic Mastocytosis with an associated hematological neoplasm (SM-AHN) is a variant of SM associated with another hematological neoplasm, such as the myelodysplastic syndrome, myeloproliferative neoplasm or acute myeloid leukemia.1 In spite of this, Mast Cell Leukemia (MCL) is rarely associated with clonal hematologic disease.2,3
DescriptionThis was a 51-year-old female patient previously diagnosed with myelodysplastic syndrome with ring sideroblasts (MDS - RS) since July 2004. At the occasion, she presented with macrocytic anemia (Hb 10.7g/dl, Ht 31.3%, VCM 112fL) and normal white blood count and platelets. A bone marrow analysis confirmed myelodysplastic founds and a morphology analysis, 64% of ring sideroblasts. The karyotype was normal. She was followed from 2004 to 2008, became asymptomatic and kept hemoglobin levels higher than 9.0g/dl, without other cytopenia.
From 2008 to 2011, hemoglobin levels dropped to less than 7.0g/dl, and erythropoietin treatment was required. From 2011–2015, the clinical course worsened with asthenia and need for blood transfusions. At this point, bone marrow evaluation showed dysplastic findings, but no mast cell disease. She received four cycles of azacitidine without improvement. An abdominal ultrasound in 2015 showed discrete hepato-splenomegaly.
At the beginning of 2017 she experienced a clinical deterioration, needed blood transfusion every fifteen days (she had more than 30 blood transfusions) and evolved with increased hepato-splenomegaly (liver 12cm and spleen 8cm from the ribs). The blood count dropped, with hemoglobin between 4.8–6.0g/dl, even with blood transfusions.
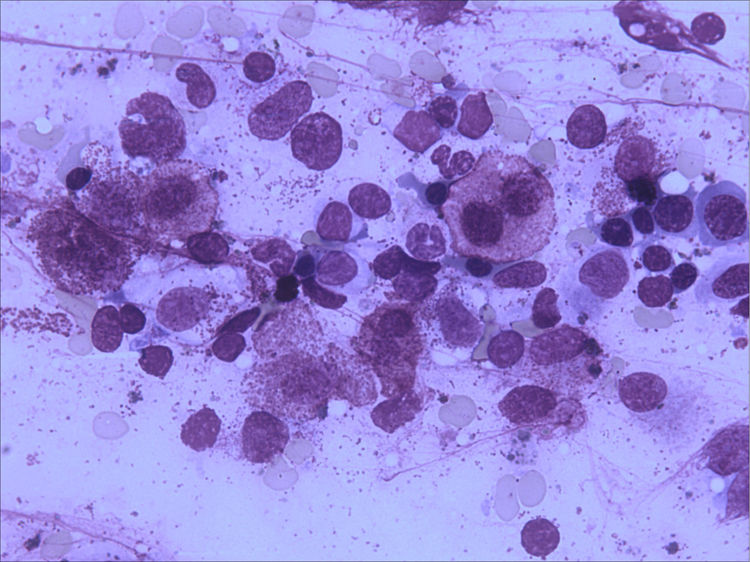
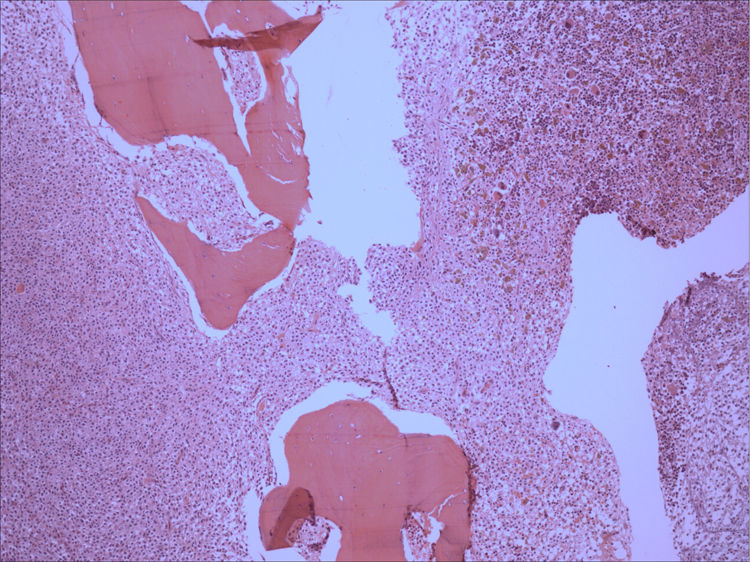
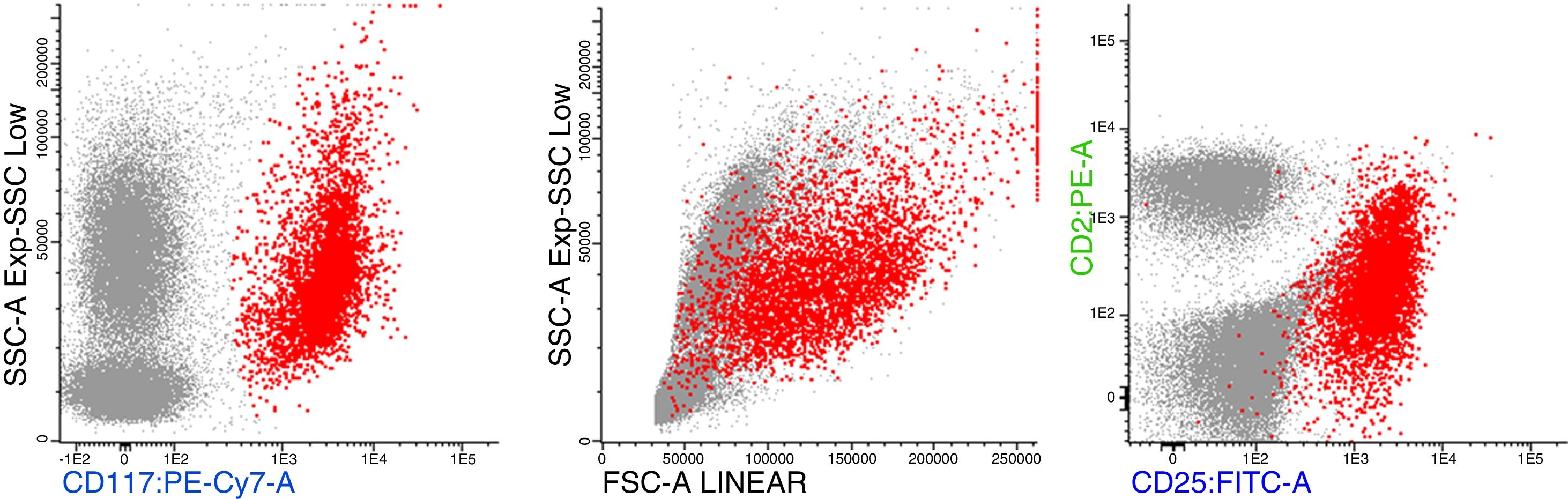
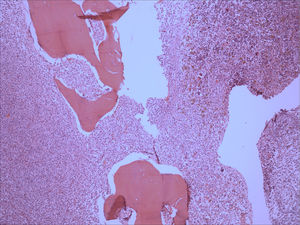
Bone marrow aspirate was infiltrated with 20–30% of mast cells, round rather than spindle-shaped, with hypogranular cytoplasms, and the presence of mast cells clumps, one of them with more than 27 cells (Fig. 1). A bone marrow biopsy confirmed a massive mast cell infiltrate with immunohistochemistry positive for CD117 and the presence of grade 2 fibrosis (Fig. 2). The evaluation of iron stain showed a high bone marrow iron score and more than 50% of ring sideroblasts, confirming the persistence of the myelodysplastic syndrome. She had no skin lesions and peripheral blood did not show atypical mast cells. Multiparametric flow cytometry confirmed the presence of abnormal mast cells (CD117 and CD45 positivity) with strong positivity of CD2 and CD25 antigens (Fig. 3). Mast cells were negative for T and B cell antigens (CD3, CD4, CD8, CD19, CD20 and CD10), myelomonocytic antigens (CD11b, CD13, CD14, CD15, CD64) and precursor antigen (CD34). The molecular analysis of the D816V c-kit mutation was negative. The triptase level was not available.
This patient meets the criteria diagnosis for aleukemic mast cell leukemia, according to the World Health Organization. She received imatinib mesylate 100mg/day for three months without clinical improvement and was referred to receive bone marrow transplantation. Unfortunately, she was admitted at the intensive care unit (ICU) with diarrhea, fever and acute respiratory distress syndrome. A hepatomegaly with ascites and a big splenomegaly (spleen 21cm for ribs) were present. At this moment, the mast cell activation syndrome was suspected because of the presence of nausea, hoarseness, vomiting, diarrhea, hypotensive syncope and tachycardia.4 The patient died with multiple organ failure five days after entering the UCI.
Discussion
According to the World Health Organization (WHO) classification, advanced systemic mastocytosis (SM) comprises patients with aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) and mast cell leukemia (MCL).1 MCL can occur as de novo, or secondary to other mast cell diseases,1,2,5 and has a particularly poor prognosis with median survival time of less than six months. In addition to meeting the 2016 WHO criteria for SM, a diagnosis of MCL requires a bone marrow biopsy showing diffuse infiltration by atypical, immature mast cells and/or bone marrow aspirate smears showing more than 20% of mast cell. In classic mast cell leukemia, the mast cells account for more than 10% of peripheral blood cells, but the aleukemic variant is the more common form. Skin lesions are usually absent.1
Flow cytometric analysis of neoplastic mast cells reveals detection of aberrant CD2 and/or CD25 expression by CD117/kit gated mast cells.1,6 Mutations of the gene coding for the c-kit receptor (mutation KIT(D816V)), leading to constitutive signaling through the receptor, are found in more than 90% of patients with systemic mastocytosis and approximately 50% of those with MCL.1,7 It has been established that the presence of this mutation confers a resistance to imatinib, but this drug may affect SM with other sporadic mutations.1,8 Recent data have highlighted that the molecular pathogenesis of advanced SM is complex, with one or more additional mutations.3,4
Systemic mastocytosis with an associated hematological neoplasm (SM-AHN) is a variant form of SM associated with hematological neoplasm, which occurs in 40% of the cases.2,9 Despite this, MCL is rarely associated with clonal hematologic diseases. Less than ten cases of MCL with associated hematologic neoplasm were found in previous literature.1,10–12
Recently Jawar et al.4 evaluated clinical and molecular characteristics of 28 patients, with (n=20, 71%) or without an associated hematologic neoplasm. The de novo mast cell leukemia was diagnosed in 16 of 28 (57%) patients and secondary mast cell leukemia evolving from other advanced systemic mastocytosis subtypes, in 12 of 28 (43%) patients, of which 7 patients progressed while on cytoreductive treatment.4
Effective treatment is not available for patients with MCL and the prognosis is very poor, with a median survival time of less than six months.12 Midostaurin might be an option for MCL, but overall response is not higher than 42%.4
The mast cell activation syndrome (MCAS) is a condition characterized by mediator-related symptoms associated with a substantial systemic activation of mast cells and can be suspected when typical clinical signs of severe recurrent acute systemic MCA are present and the symptoms respond to treatment with MC-stabilizing agents (anti-histamines).13
This case illustrates the rare association of mast cell leukemia with low-risk MDS-RS, which pursued a fatal clinical course with catastrophic MCAS and death.
Conflicts of interestThe authors declare no conflicts of interest.